Going back in time
Interview with
Neural stem cells in injured axolotls behave like embryonic cells. This enigma of neuroscience has stood for decades; Elly Tanaka has been taking a genetics approach to try to get to the bottom of why this happens...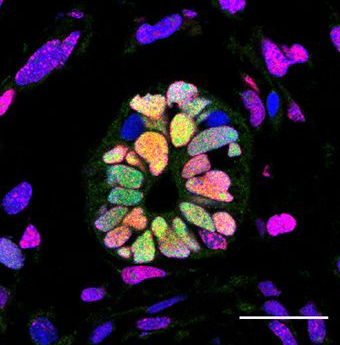
Elly - We’re trying to understand why the salamander can regenerate these very complex body parts like the limb and the tail. Remarkably, within the tail a fully functional spinal cord can be regenerated after we amputate a piece of the tail off. So, they have some cells as we have stem cells in the nervous system but signals that get made after the amputated tail promote these stem cells to build a new spinal cord and that’s what we’re starting to study now.
Chris - Why does the same thing not happen in us though? We’ve got stem cells too. Why don’t I grow a new spinal cord if I injure it?
Elly - Many people think that there are differences in the immune system and the immune reaction. The study that we have done in this case indicates that the neural stem cells in a salamander can go back in time and become like a stem cell that you find in a very early developing embryo. And so, our hypothesis is that in humans, this kind of what we call de-differentiation or the ability of adult cell to become an embryonic-like cell again doesn’t happen.
Chris - So something stops the cells un-specialising which is what enables them, endows them with the ability to almost think they're back creating an embryo again and regrow a structure from scratch.
Elly - Right.
Chris - Now how do they know “How Big” the tail they’ve got to make is, given that when they were growing in an embryo, they may be in order of magnitude or more smaller than ultimately the adult thing that they turn into?
Elly - That’s a very interesting question. We’re working on that now in the lab. It seems that the cells surrounding the spinal cord might give the instruction as to where along the body you are then there's some kind of system for saying how much you have to grow. But in terms of this issue about size, the embryo is tiny and then an adult x level is huge. It seems that when a spinal cord regenerates, you turn on a subset of these embryonic genes and this allows the existing spinal cord to just extend itself using mechanisms that stimulate stem cells to divide in a specific orientation so that you extend the two rather than making a big round mass of cells.
Chris - Yes, that was going to be my follow up point which is also, it’s got to know again, which direction it wants to grow in and only grow in that direction. So, how do you think that’s achieved?
Elly - When you amputate the spinal cord, the tip cells, they start to make healers that basically fill out into the open space. We believe that kind of initial orientation is probably very important in orienting a process called planar cell polarity that tells the two which direction is kind of the backend, so in which direction to grow. Then the cells in the spinal cord then start dividing in that direction. So they orient their division apparatus so that both daughters end up pointing in the direction that the spinal cord should grow.
Chris - They inherit a direction of recovery from the original injury which polarises the cells. They know what direction they have to grow in and at the same time there are signals coming in laterally. In other words, from the side, from other tissues which gives them a clue as to how far down the body they are so they roughly know how many divisions to go through in a longitudinal direction to rebuild the tail.
Elly - Yes. So that lateral communication between the surrounding tissue and spinal cord, there are some general experiments that indicate that that’s happening but there's very little known about that process.
Chris - But how do you know that the changes that you're seeing in these gene expression profiles are upstream of the developmental and regenerative outcome and they're not merely a bystander effect that they are actually orchestrating this process? Have you tried manipulating the levels of those genes and then demonstrating that there is a different regenerative outcome when you do that which proves they're necessary and sufficient to have that outcome?
Elly - Yes. That’s one of the important things of the study. We deregulated this pathway called planar cell polarity which cause the stem cells to divide in the wrong orientations. By doing that, we blocked the spinal cord from growing out. When you do that actually, the cells, they don’t expand enough and they start turning into neurons too early.
- Previous Up in the air
- Next Food for Thought










Comments
Add a comment