This month we're taking a trip into the world of developmental genetics, finding out how an animal grows from one cell into many millions as it develops from a fertilised egg, and discovering how it knows when it's grown enough. We also hear about the hunt for genes involved in autism, see what sticklebacks can tell us about evolution, ponder the purpose of keeping 9,000 placentas, and ask whether we can ever genetically engineer humans to drink seawater. And the monster raving loony gene of the month is the wacky-sounding Lunatic Fringe.
In this episode

01:21 - Developmental genetics - Dr David Ish-Horowicz
Developmental genetics - Dr David Ish-Horowicz
with Dr David Ish-Horowicz, Cancer Research UK London Research Institute
David - In the case of the mammalian embryo, probably the first decision that's taken is when there are enough cells that some of the cells are on the inside, and other cells are on the outside, those two types of cells become different. And the outside cells become kind of accessory cells that aid the inside cells to form the actual mature animal. In the case of other animals, we work a lot on fruit flies. You actually start off by making an embryo which has a few thousand cells and at that point, actually, the embryo then knows its front from its back, and its top from its bottom. And it also knows that it's going to make a segmented, a repeated animal, and so, from then on, it refines these initial asymmetries into what ultimately becomes a very complicated animal. You might think a fly is simple, but in fact, when you look at it in detail, it's pretty complicated, and it's just as difficult for us to envisage how you make a fruit fly as how you'd make a human being. It's just a matter of scale and indeed, what's been exciting over the last 30 years or so is the discovery that the genes that are important in making a fruit fly are actually the same genes that are important in making a human being.
Kat - And that's quite crazy because they are such different processes. I remember if I cast my mind back that the fruit fly egg has a front and a back, and it's very organised whereas a mammalian egg looks a bit different and a bit less well-organised, but you were saying that it's actually the same genes that are controlling the subsequent process after that.
David - The same genes are always used, but not always in the same way because there's only a limited repertoire of genes. So, you use them again and again, and they do different things in different contexts. I think the fruit fly is unusual because quite a lot of its patterning is done in the mother as an unfertilised egg whereas in the case of a human embryo, most of that patterning happens after fertilisation. I think what it comes down to is that because you only have a limited number of genes, and a limited number of ways in which they interact - you've got a limited number of building blocks, but you can use the building blocks in many different ways. Just as when you build a house, you may have a fixed set of components, but you can build radically different houses. The same is true of animals and quite subtle differences in timing can make apparently big differences in the final appearance of the animal, but actually, underneath it all, it's really not as different as you might think. I mean, at the end of the day for example, if you have a muscle cell, what a muscle cell does is it takes chemical energy and converts it into movement and once evolution had worked out a way of doing that, it stuck with it because that's what you do. You use what works. So obviously, there are differences between muscles in flies and in humans, but there are massive similarities in the way they work as well.
Kat - Tell me a bit about what you've got going on in your lab at the moment. What questions are you really trying to get to the bottom of?
David - I suppose the thing we're most interested in are the asymmetries. The flies are a particularly strong example whereby the asymmetry of a final animal, the complicated architecture is anticipated by asymmetries very early in development and in fact, by asymmetries within a single cell. So, we're interested in how a single cell can have a front and a back, and a top and a bottom. What are the cues that set up these spatial differences? And that turns out to be the putting of particular chemicals in particular places when you're building the cell. And so, you can put one particular chemical at the front of the cell and a different chemical at the back of the cell, and now, you've got the difference between the front and the back, and so on and so forth. We're interested in the different kinds of mechanisms whereby that can happen. One of those mechanisms in the fly is actually to use little molecular motors that take energy and move molecular cargoes to particular places in the cell where they are needed. Now that's a particular use of molecular motors. In fact, they're used very widely throughout development to put things in the right place.
Kat - So, it's an incredible mental image of a tiny, tiny cell, and all these motors scuttling around, delivering things to one end, to the other end, and then as it begins to divide to make an embryo, you're separating off these different types of cargo from different ends.
David - It is indeed very exciting and one of the breakthroughs in the last 10 to 15 years is that microscopy has allowed us to actually see these movements. I mean, the advances in microscopy means we can image where things are and watch them move.I mean, it's aesthetically very beautiful to see some of the time-lapse movies that people can make, things moving around cells, just to be placed in the right position to work.
Kat - And these are in living, developing organisms?
David - Indeed, you can now do it - you used to just have to study things in animals or the tissues when things were dead but increasingly, we can look at things actually happening because the sensitivity of the microscopes and the resolution of the microscopes, and the techniques of labelling molecules so that you can see them have improved beyond recognition.
Kat - Where next for this field? What do you think are the big questions that we still need to figure out?
David - We know a lot of the components that are involved. I mean, we know the gene sequences and we know a lot of the words involved in building the book that's an animal because we know the genes, and we know what proteins they make, but we don't necessarily know what the proteins do and we don't necessarily know how they work together. And so, I think a lot of the logic behind it is still to be discovered. Exciting things for the future I suppose are to take what we know already and extend them amongst other things into how the nervous system works, the real logic behind the very, very complicated circuitry in even a fruit fly brain. That I think is going to happen gradually over the next years. We know so much more than we thought we would know 20 years ago, but we're still scratching the surface about many other things.

08:51 - Autism genes
Autism genes
with Nell Barrie
Nell - So the first one is looking at the genes behind autism and this is research in America and they've looked at about 600 families who've got people with autism in the family, and they actually found three new high risk genes. But it also looks like the whole disease is a lot more complicated in terms of which genes are contributing than we thought before.
Kat - Because I think it was thought there's about 200 genes involved in autism, but now, this new study puts the number at about a thousand. That seems very complicated.
Nell - Yeah, exactly and I mean, it's a behavioural disease so it's kind of what would be expected I suppose, that there's lots of different changes that can maybe cause the same types of symptoms in people, and they've actually looked at gene mutations that are not inherited from the parents, but are just arising spontaneously, so just little changes that happen by accident randomly.
Kat - So this is more likely to be relevant to autism and the whole population then rather than kind of specifically inherited syndromes, do you think?
Nell - Yeah, it sounds like that and I mean, I guess it's another one of those things where you can look at all these different changes, figure out how common they are as a background sort of thing, and then perhaps that will give you clues for ways to treat this and ways to tackle the problem.
Kat - Certainly, important because there's more and more people being diagnosed with autism. I think a lot of that is they've changed the criteria for diagnosis and more people are falling within that spectrum disorder. One of the interesting things and the challenging things I saw, I think in the press release, one of the researchers says the phrase, "The bad news is, there is heterogeneity out the yingyang..."
I think they've just found this huge number of genes and it's very complex. It's all sorts of different versions of them, so it's a really big challenge and how do you think they might straighten out?
Nell - Well I guess, I mean, one thing I thought was interesting is that could perhaps be part of the reason why you see this sort of spectrum of autism-like disorders I guess. You've got some people who are really badly affected, others who maybe just have some symptoms, and perhaps that could give us some clues as to what's going on there.
Kat - I think larger studies as well, just to try and figure out really what's going on a much larger basis.
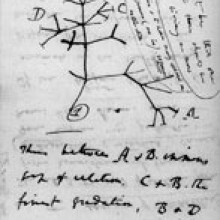
10:56 - Rebuilding evolutionary trees
Rebuilding evolutionary trees
with Nell Barrie
Kat - Another interesting paper that I noticed in the American Journal of Human Genetics, talking about a Copernican revolution in how we view where we start comparing DNA sequences from, what do you think about this story?
Nell - Yeah, so this is very kind of revolutionary sounding in the way they talk about it which is nice and they're looking at mitochondrial DNA and how you should start comparing mitochondrial DNA from now, mitochondrial DNA from the past, should we just be using mtDNA from white people or should we be looking wider, and figuring out what exactly is going on, and building up the data that we have I suppose.
Kat - I think this kind of stems from a lot of the genetics research that has been done over recent years has all been done using the samples that we can get which tend to be white European DNA. And a lot of the genetic trees and work that's been done has kind of almost assumed that this is the centre of the genetics universe and the argument here they're making that is mitochondrial DNA which comes from mother's eggs and is passed on. Every time we compare it and maybe normal genes as well should really start by looking at ancestral DNA and comparing forwards from that. There's a lot of rhetoric in the story about, you know, it's a Copernican revolution and the world should not necessarily revolve around white male European DNA. But yeah, I thought it was interesting to propose that for researchers, just the way they think about how they build trees.
Nell - Yes, and definitely now that it's so much easier and quicker to do all these kind of sequencing that's going to have a big effect and I suppose it just means you're opening up a whole new area where you can do more research, and find out more and use more of that information.

12:38 - Stickleback evolution
Stickleback evolution
with Nell Barrie
Kat - Another story that we saw, also involving this large scale genome sequencing but not in humans, but in fishes. What's this one?
Nell - Yeah, I like this. This is about sticklebacks and how they've evolved and they were actually looking at how the different species evolved to adapt in the same sort of way, and it's a sort of convergent evolution thing. So they haven't all come from one common stickleback ancestor. They've all adapted similar genes in similar ways, but independently which is really cool.
Kat - So it's like 21 different species of sticklebacks that they sampled from different lakes around the world that obviously, they separate and they're separated by geography. But yeah, they found that the genes are changed in the same way to help the sticklebacks adapt so I thought that was a really nice example that tells you about how evolutionary processes might work. There was quite a sad thing I saw as well.
Nell - Yeah, at the end, they were saying that some of the sticklebacks they've been studying from a place called Bear Paw Lake were actually being threatened by some big pikes that are coming in which are invasive predators. I mean, you might think, "Oh, it'll be fine. They can re-evolve themselves" but we know that that's not true and every species is individual and unique, so yeah, they have a bit of a sad end to that.
Kat - They can't evolve. They've all been eaten.

13:54 - Children of the 90s
Children of the 90s
with Nell Barrie
Kat - And finally, there's a story that I saw basically that starts talking about 9,000 placentas floating in plastic buckets of formaldehyde...
Nell - Yeah, who wouldn't read on after that. I think that's great.
Kat - This really got me and this is about the Bristol cohort. What is this here?
Nell - So there's a slightly more kind of evocative name which is 'The children of the '90s' and this was loads and loads of children, more than 4,000 that were kind of selected to be part of this massive cohort study. And they basically wanted to find out every possible thing they could about how these children started off in life, about their mothers, about their development as they grew up. So they've got all kinds of crazy information and tissue samples and stuff stored away that they're now finding exciting new ways to use.
Kat - So they've got like placentas and hair, and they've got all sorts of data, and some of these kids are now - 20 years later, they're now having children themselves, but what I think is really pioneering about this is that they were collecting samples and placentas, all those kind of things, before they really knew what they could do with it. Because back then we didn't have the kind of genome sequencing technology we have.
Nell - Yeah and I mean, there were some quite nice old stories within this about, "what kind of things should we ask the parents about" and it was just like, "Let's ask them everything. Have you got a tumble drier, have you got a dog, do you use pesticides in the garden?" It was just kind of anything that might possibly be relevant even in ways that they couldn't imagine at that time. So I think yeah, it's really kind of forward-looking research because they just didn't know how they're going to use this stuff, but now, there's lots of different ways that they could.
Kat - It's really blue skies. I love the idea of someone going, "This will be useful one day." It's like your mum going, "Just take that and put it in a safe place." You know, 9,000 placentas, keep an eye on that. Sure it would be useful one day. You haven't got any placentas at home?
Nell - No, although I am a bit of a hoarder so you know, you'd never know when things might come in handy and I think that's what science is all about.

15:52 - Head lice vs body lice
Head lice vs body lice
Scientists from the University of Illinois have found more evidence to suggest that human headlice and body lice may be the same species - something that's hotly debated in the world of insect genetics. The researchers compared the activity of genes in both types of lice throughout their life cycle, and subjected them to a range of challenges, such as heat or bacterial infection. They found so few differences between the two that they could arguably be the same species. Interestingly, body lice can transmit diseases like typhus while head lice don't, so the next step is to try and understand how this difference exists when the two species are so similar.

16:33 - Huntington's disease and cancer
Huntington's disease and cancer
A paper from Swedish researchers in this month's edition of the Lancet Oncology shows that people with Huntington's disease and other similar conditions have a lower risk of developing cancer than the general population. Certain neurodegenerative diseases like Huntington's are caused by gene faults that lead to an excessive buildup of specific proteins in nerve cells. At the moment it's not clear exactly how this leads to a reduced risk of cancer, but the scientists hope it could shed light on some of the molecular pathways that underpin the disease and even potentially lead to ways to prevent the disease in the future.
17:08 - Birth defects
Birth defects
An international team of researchers have made an important step forward in understanding how nature and nurture work together when it comes to birth defects linked to faulty genes, according to a new paper in the journal Cell. The scientists were studying a relatively rare disease called congenital scoliosis, which causes babies to be born with a poorly-formed spine.
Using mice with a gene fault that mimics the human disease, the team found that even short periods of low oxygen during pregnancy (known as hypoxia) increased the risk and severity of baby mice being born with spine problems. In humans, hypoxia can be caused by a range of things like maternal smoking, high altitude, drug use or medical conditions such as maternal diabetes or anaemia, so the researchers think that the same mechanisms may be at work in human babies, increasing the chances of birth defects where there's an underlying genetic defect.
18:01 - Alzheimer's genes and brain development
Alzheimer's genes and brain development
And finally, new studies published in the journal Nature Genetics have shed light on the genes involved in Alzheimer's disease and brain development. Overall, the research involved more than 80 scientists at 71 institutions in eight countries. The first study, based on genetic data from than 9,000 people, discovered that the hippocampus - the brain region involved in making new memories - may shrink faster in people with certain versions of four genes, increasing the chances of developing Alzheimer's.
The second study found two genes that help to control brain size, by affecting something known as intracranial volume - the space inside the skull that the brain fills. As well as providing important clues about brain development and disease, the scientists also think these genes may have played a role in human evolution, helping us to become the brainy creatures we are today.

19:12 - How do we know how big to grow? - Dr Nic Tapon
How do we know how big to grow? - Dr Nic Tapon
with Dr Nic Tapon, Cancer Research UK London Research Institute
Nic - If you look around you, you look at animals in general, each species has a specific size and shape and there's not - I mean, there is some deviation from that specific size and shape, but by and large, they're all roughly the same size. It's a very interesting question how that's achieved time and time again as the animals undergo development and have to grow from a very small embryo to a very large adult, how they precisely know when they've reached the appropriate size and shape.
Kat - So we don't see fruit flies the size of birds, we don't see Basset hounds the size of mice.
Nic - Yes, that's exactly right. So, this question has very interesting evolutionary implications because related species vary quite considerably in size and shape. If you look at dogs for example, different species of dogs, and even with related species like wolves have very similar genetic material. So they're very similar at the level of their DNA and yet, their size and shape varies amazingly and so, that's a particular situation where very small changes in the genetic makeup of the species really has a huge influence.
Kat - Presumably, there are genes that are responsible for this. What do we know so far about the kind of genes that are controlling an organism's size?
Nic - Yeah, so there are many different genes that have been found to regulate size. The particular genes that we're interested in belong to a signalling pathway, so it's a pathway that tells the cell how much to grow and to proliferate, and that pathway is called the Hippo pathway. So it was discovered in flies and the reason it is called the Hippo pathway is not because the fly gets to be as big as a hippo, but the mutants are actually much larger and they become very wrinkly, a little bit like the skin of the hippo because there's so much tissue that the cells don't know where to go. And so, that particular Hippo pathway we think is influenced by many different factors really in the developing fly which allows it to sense tissue size.
Kat - What are some of the issues that influence how big an organism grows and how it controls its size?
Nic - One particular issue is nutrition. So, as you know, we are what we eat and during development, how much nutrition the organism receives has a very strong influence on overall size. And so, the Hippo pathway we think does respond to nutritional cues. In flies obviously, that's quite a big deal because as you can imagine, flies are very much exposed to their environment and very often, they don't get enough food or the larvae don't get enough food as they're developing. And so therefore they need very potent mechanisms to couple nutrition intake or availability with growth during development. The other type of mechanisms that regulate the Hippo pathway are to do with really the differentiation of the tissue itself essentially. So, they need to differentiate into that particular cell fate. This differentiation process is coupled to growth because when you become differentiated then you stop growing and dividing, and so, the Hippo pathway we think also is influenced by the differentiation status of the cells.
Kat - So, does this help to explain why a liver is always more or less the same size in relation to the rest of the body, your kidneys are always the same size compared to the rest of your body too?
Nic - Yes, that's right. So this is a process called 'allometry' which is that you need to coordinate the growth of all the organs in our bodies so that the proportions are respected, so you can't have somebody going around with huge liver and a tiny brain. That wouldn't work very well and so, that needs to be coordinated, and that's probably done by the organs communicating with each other. This is something that we still know relatively little about, but it's a very fascinating process both during development, but also in adults because remember that growth control is not just about reaching the right size which happens as you're developing from an embryo to an adult. But also, once you're an adult, you have to maintain, you have to keep sensing the maximum size and you have to maintain that, and that's a very interesting process as well because it relates to diseases such as cancer for example where those process' balance which is called homeostasis, goes wrong and you start to get tumours.
Kat - For you and your research, where are you headed next? What are the questions that are really bothering you?
Nic - We have many of the players that control growth. So the signalling pathways, we've identified them but we still don't really understand the precise cues that these pathways are responding to, so in particular, in the Hippo pathway, what we think or for the case of the Hippo pathway, what we think is that it responds to the physical environment that the cells are in. So, as you start growing, the kind of tension, the pulling and pushing forces that are sensed by your cells change. The force balance within your body changes as it gets bigger and we think that this is precisely what is being sensed by the Hippo signalling pathway and that once the force balance kind of changes in a particular way then the cells are told to stop proliferating because you've reached the right size. But that's a very vague concept as you can imagine and the tools for us to actually probe these forces - we're talking about really physical forces here, pulling, pushing, and the tools to probe these forces are still in their infancy and particularly, if you're looking at a system which is alive, which is a living system. And so, I hope in the future, we will develop better tools to visualise physical forces in developing tissues, and that will tell us really how growth regulation is happening.
Kat - Because it's very easy to think about nutrition, and chemicals, and glucose or whatever, affecting something but to get a handle on what must be tiny atomic scale forces, what sort of technologies are you starting to look at?
Nic - Well, for the moment, it's mostly to do with ablating little bits of tissues with lasers which we can do in cultured drosophila embryos and that's certainly works, but it's a very crude tool. It doesn't really give you kind of very good handle on the exact extent of the forces. It can give you relative measurements which is useful, but what the future is made of is really using biosensors. So you've probably heard of biosensors in every context as these are essentially reporters of particular physical processes - either physical or signalling processes inside the cells that are big business in the pharmaceutical industry as they're used to probe the function or the activity of drugs for example on our cells. We and others are developing biosensors of physical forces that should enable us to really in situ or in vivo examine these forces, but there are still - part of me wishes that I had a Star Trek tricorder, one of these lovely little devices that you just flash at someone and you get all of their physical parameters instantly downloaded onto your computer. But unfortunately, Mr. Spock hasn't given me one of these yet.

Could genetically engineered humans drink seawater?
Obviously this would be quite useful as there are many places where it's hard to get freshwater, and most of the water on the planet is salty. Unfortunately we dehydrate very quickly if we drink it on its own, because our kidneys have to get rid of more water than we would actually take in from the seawater to get rid of the extra salt. This happens because we can only make urine with a certain level of saltiness, because the tubes in our kidneys that concentrate urine, known as the loops of Henle, are of a fixed length. Some animals like the desert rat can drink relatively salty water, as they have very long loops of Henle and can produce very concentrated, salty urine. So if we could find out the genes that affect the length of these loops, I think it's theoretically possible that we could genetically engineer humans with kidneys that can cope with drinking saltier water. But because the kidneys develop very early in life, you'd have to do this while a baby was growing in the womb. And this kind of genetic modification is technically and ethically very difficult to do.

Can radiation cause beneficial mutation?
The short answer is "yes!" - although large doses of radiation from something like an atomic bomb or nuclear power station explosion are obviously very bad, any type of lower-level radiation doesn't cause specifically "good" or "bad" mutations, it just causes changes. As we heard in last month's podcast, these random changes happen all the time and may or may not be passed on to the next generation. But if there is a selective pressure, then we see beneficial mutations start to spread through populations. It doesn't matter what causes them, only that they have to be passed on, so they'd originally have to arise in the sperm or egg-producing cells for humans and other animals.

30:29 - Gene of the month - Lunatic Fringe
Gene of the month - Lunatic Fringe
with Kat Arney
Finally, keep your hair on, because our gene of the month is the rather wackily-named Lunatic Fringe. First discovered in fruit flies, the Fringe gene is involved in forming the edges of a fly's wings. Mammals - including mice and humans - actually have three different versions of the Fringe gene, called Manic Fringe, Radical Fringe and Lunatic Fringe, which are involved in the development of the limbs and other parts of the body.
In 2006, scientists discovered that inheriting two faulty copies of Lunatic Fringe causes severe problems with the development of the spine - a condition known as spondylocostal dysostosis, which comes under the banner of a number of hereditary spinal defects known as Jarcho-Levin syndrome. Although the name Lunatic Fringe sounds amusing, it's much less funny to families whose children have been born with the syndrome, so the Human Genome Organisation Gene Nomenclature Committee, or HUGO for short, have suggested it should be known as just LFNG. Less creative maybe, but probably also less upsetting.










Comments
Add a comment