FOXG1 Syndrome: Fighting the Odds
Listener Vivek got in touch with a question about a rare genetic disease his son has, called FOXG1 Syndrome. In fact, it's so rare - and so newly-discovered - that only about six hundred people in the world have been diagnosed. Kids with FOXG1 have severe developmental delays; in Vivek's words, "everything that can go wrong - it's gone wrong with him." But the parents of FOXG1 children have been unusually tenacious when it comes to shaping the course of science. In this programme we meet those people blurring the line - metaphorically speaking - between the brain and the heart.
In this episode

00:09 - Vivek and Kush
Vivek and Kush
Vivek Singha, FOXG1 Foundation Australia
Recently, Phil Sansom had a listener called Vivek Singha get in touch...
Vivek - I came across a couple of your tweets and that's why I reached out to yourself.
Phil - Vivek was asking us about testing for rare genetic diseases - in particular a disease called FOXG1.
Vivek - I'm a father of a child who was diagnosed with FOXG1 syndrome. Kush was the first kid in Sydney who was diagnosed.
Phil - In fact this condition, FOXG1 Syndrome, is so rare - and so newly-discovered - that only about six hundred people in the world have been diagnosed.
Vivek - It's a gene on chromosome 14, a small mutation. Causes profoundly debilitating impacts on the kids. One way to look at it is like on a 20 kilometre long piece of string, there's a one millimetre gap; and that one millimetre gap is causing issues for Kush. His early development has been hit really hard. He was diagnosed with cerebral palsy; quadriplegic, so he's unable to control either of his four limbs; his vision, his brain can process the vision he's getting through his eyes, so he's really hit hard from a vision perspective as well; his arms and limbs keep moving all the time, so it's hard for him to voluntarily control his movements. It's a terrible, terrible set of... everything that can go wrong, it's gone wrong with him.
Phil - Vivek’s son Kush isn’t getting worse, but he has had his development delayed. As we spoke, I found out that while Kush is a great kid, things have been really rough - for both him and his parents.
Vivek - When he was diagnosed at a very young age, it took us a long time just to accept the diagnosis as well. Like, I wouldn't wish it on my worst enemy.
Phil - No genetic disease is ever going to be a walk in the park, of course. But as I started looking more into FOXG1, I found out that something about it has inspired Vivek - and others like him - to roll up their sleeves and actually start to shape the course of science. In this programme I meet those people blurring the line - metaphorically speaking - between the brain and the heart.
Vivek - We've got four kids, Kush is the youngest. I want Kush to be able to run like other kids as well.
02:27 - What is FOXG1?
What is FOXG1?
Hannah Bruce, UCL; Tim Rittman, University of Cambridge
Until 2008, the rare disease FOXG1 Syndrome was being misdiagnosed as a severe version of something called Rett Syndrome - another neurodevelopmental disorder with similar symptoms. But the truth is that underlying FOXG1 Syndrome are problems with the specific FOXG1 gene - according to UCL researcher Hannah Bruce...
Hannah - Under normal circumstances, you have two copies of FOXG1: you get one from your mom and one from your dad, but in a particular disease case called FOXG1 syndrome, you only have one working copy of FOXG1. The FOXG1 gene encodes information to make a protein, which is basically a machine that does a lot of work in your cells.
Phil - And what does that protein then do?
Hannah - The FOXG1 protein is really cool, I think. It's first job is to control the expression of turning on and off of other genes.
Tim - FOXG1 in particular is what we call a transcription factor; that is involved in turning different other genes on and off.
Phil - Like a master control switch or something?
Tim - Yeah, that's a really good description, yeah. So it controls all these other genes.
Phil - That’s Tim Rittman from the University of Cambridge. I went to see him to try and visualise what the FOXG1 protein actually does - and it’s a vital job for your developing brain that starts in those first few months in the womb.
Phil - Can you help me visualise which parts of the brain this is actually helpful for?
Tim - Yeah, of course. We've got a model here of the brain...
Phil - And it's a lot bloodier than I normally see a brain!
Tim - Yeah, so the brain has got this amazing network of blood vessels running all over the top of it. And you're right, this particular model has got all of those blood vessels on.
Phil - I can understand why they don't normally show it, because it's quite disconcerting.
Tim - It's a little bit gory, isn't it?
Phil - Which parts of the brain is FOXG1 going to be most important for?
Tim - So the brain looks a bit like a sort of walnut, really, with all those folds. That cortex is where FOXG1 is really important.
Phil - The top part.
Tim - That's right, yeah. And if we open the brain up, you can just see in the middle here...
Phil - You've given me a cross section here, and it's like the centre of the walnut.
Tim - Just about as far as down in the brain as you can get.
Phil - This set of relatively small lumps is called the basal ganglia.
Tim - So those two things together, these basal ganglia and this cortex, is where FOXG1 is really important.
Phil - Together the cortex and the basal ganglia are called the cerebrum, the top part of your brain. It’s actually the biggest part, and it’s useful for what you might think of as higher human abilities - emotions, complex intelligence, and memories. It includes everything except for some parts near the bottom. And so when you have a problem with FOXG1, all those important areas don’t develop right, and you end up with a visibly smaller brain. The other big issue you face involves a crucial part in the middle of this cerebrum...
Tim - Now of course we've got two hemispheres here, left and right, and there's a really important connection between the two: this C-shaped structure here just below the cortex, just above the basal ganglia. And that is called the corpus callosum.
Phil - It's like a layer between the centre of the walnut and the crinkly, walnut-y part.
Tim - This is the main communication between the left and the right side of the brain. So this is like a massive, huge bunch of telephone wires essentially. And when we see people with FOXG1 problems, that corpus callosum is affected and a lot thinner than it should be. So there's less messages getting from one side of the brain to the other.
Phil - And it’s not like the two hemispheres work fine on their own. These messages are crucial for most of the things the brain does.
Tim - You take something like language, which usually you'd find in the left hemisphere; it's still got to communicate with the right hemisphere, which has some of the same function in it. So this connection between the two is absolutely critical for all of our brain functions, really.
06:11 - FOXG1 fish - and their glowing brains
FOXG1 fish - and their glowing brains
Hannah Bruce, King's College London
On the spectrum of important genes for development, FOXG1 is high. In fact, it used to be called Brain factor-1; that’s how big a role it plays in your brain. Phil Sansom spoke to Hannah Bruce, one of the people studying that role...
Hannah - In science, it's not always feasible or ethical to use humans in studies. Instead we like to use models, model systems, to try and understand more about diseases. So when I say a model, I'm using the zebrafish to model FOXG1 syndrome. Because I'm not able to do experiments on the children with FOXG1 syndrome, of course, that would be terrible.
Phil - What's a zebrafish?
Hannah - A zebrafish is this tiny little fish. It's maybe about an inch long.
Phil - Are they cute?
Hannah - They are quite cute. Yeah. They have little stripes just like zebras. Clue's in the name!
Phil - And do they have brains that are similar to human ones?
Hannah - They do have the same brain areas as we do.
Phil - So how do you test the FOXG1 in the zebra fish brain?
Hannah - I have these sort of families of zebra fish and one family, or line, as we call them, has had its genome edited so that it doesn't have any FOXG1 genes.
Phil - What does that do to them?
Hannah - The fish that don't have any FOXG1 die. They die about seven to 10 days after they're born.
Phil - Wow.
Hannah - Yeah, I know. But we can take them before they die and have a look at their brains. So one really cool thing about the zebrafish is that we can make them see-through. We add like a special chemical that stops them from developing any pigment in their skin. So I can then look down the microscope and look at the zebrafish brain while it's still alive. When I do this, the zebrafish that doesn't have any FOXG1, first of all, it has a much smaller brain, but it also has an increased number of cells that are important for excitation.
Phil - We're talking nerve cells, right, neurons, in your brain?
Hannah - That's right, brain cells. And when I say excitation in your brain, cells connect to each other. An excitatory cell will make its neighbours or connecting cells active, whereas an inhibitory cell will prevent the cells it's connected to from being active. And it's really important the number of each of those cells, or what we call the excitation inhibition balance, because if the number of those cells is skewed towards either direction, it can result in disease. So the most obvious example is probably epilepsy. The excitement of the brain is increased causing your brain to be hyper excitable, resulting in things like seizures.
Phil - Does that mean a seizure is too many nerves firing going off all at once and nothing's damping them down?
Hannah - Yes, exactly yes.
Phil - How does that relate to your poor, transparent zebrafish that die after seven days?
Hannah - What we think one of the main jobs of FOXG1 is to sort of set up the developmental boundaries where each of these cells, the excitatory and inhibitory cells, are produced. So we think it's pro-inhibitory, but it also coordinates the boundary for the production of the excitatory cells.
Phil - Oh. So if it doesn't work, then you don't get the inhibitory cells 'cause that's where it seems to be working the most strongly.
Hannah - Exactly. So the zebra fish that doesn't have any FOXG1 doesn't produce any of these inhibitory cells.
Phil - Okay. Here's my big question. Kids that have FOXG1 don't die after seven days, a lot of them live many years.
Hannah - Yes.
Phil - Are they the same as these transparent week long Zebrafish?
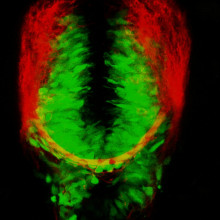
Hannah - That is a really good point. The answer is no. The children with FOXG1 syndrome, they still have one copy of FOXG1; so one of the main aims of my research is to take another zebrafish line, which only has one copy of FOXG1, to try and make it a more reliable and accurate model of the disease that we see in humans. One of the main ways that I'm trying to do this is use special zebrafish that have fluorescent proteins and their brain. Basically this means that I can have a fish that as well as only having one copy of FOXG1 is engineered in such a way that the excitatory cells glow red and the inhibitory cells glow green.
Phil - What? Like physically glow?
Hannah - Yes. Like, I can see them glowing. You should, I wish I could show you, it is beautiful.
Phil - Like Christmas lights in the fishes' brain!
Hannah - Exactly. Some people say red and green should never be seen, but I disagree.
Phil - That's amazing!
Hannah - Yeah. And one of the things that I know from preliminary research is that these zebra fish with only one copy of the FOXG1 have a decrease in the number of inhibitory cells.
Phil - And you can tell that because their brains are glowing more..?
Hannah - They're glowing, less green and more red.
Phil - Wow.
Hannah - And the really cool thing about this particular zebrafish light that I'm really excited about, is that we can use it as a fast, efficient way to read out a library of small molecule drugs. And these drugs have already been tested for safety in humans. They're completely safe to use, but the original purpose that they were to be used for, they weren't effective. And so they were basically abandoned. So what we're hoping to do is take these zebra fish that glow red and green, and pop them into single wells of a 96 well plate. So I can have 96 fish on a plate and put a different drug into each well and then readout the ratio of red to green, glowing to see if the therapy has been effective. And it's a really nice, fast, efficient way of being able to translate back from the lab bench to the bed site. Because if anything is effective, it doesn't have to go through these decades of clinical trial failures because we know they're already safe in humans and there is an effect in fish.
Phil - I'm also excited about these red, green glowing options. Have you ever turned out all the lights in your lab and had a party?
Hannah - The Christmas party? Why not? Yeah!
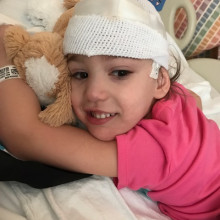
13:59 - Life with FOXG1: "I was desperate"
Life with FOXG1: "I was desperate"
Nicole Johnson, FOXG1 Research Foundation
The kids with FOXG1 only number in the hundreds worldwide, but the parents of those kids are tenacious. Listener Vivek put Phil Sansom in touch with Nicole Johnson. Along with Nasha Fitter, Nicole started her own research foundation after she wrote a blog about her experience as a parent...
Nicole - Nasha read it, and she called me and she said, "my daughter was just diagnosed with FOXG1 Syndrome. I'm also looking into seeing what research is out there. We could put a strategy together. Do you want to do this with me?" We put together a worldwide group of FOXG1 parents and we officially became the FOXG1 Research Foundation.
Phil - They fund a number of studies - which I’ll come to in a moment - all in the hopes of finding a treatment for kids like Nicole’s daughter Josie.
Nicole - Josie was born in 2011 and as far as we knew there was nothing wrong. And it wasn't really until four months that I noticed she wasn't tracking with her eyes the way my son did. And then by six months, Josie failed her six month paediatric milestone test. She just wasn't doing anything that a six month old should. It took us two years to find a diagnosis. We did every test under the sun. Full genetic testing... but they only looked for what they knew to look for, and FOXG1 just was not on the map. I went down the road of more holistic testing. I even took my family to New Mexico, and Josie, for a week, every day got needles in her head. And it didn't do anything. So I was desperate.
Nicole - So I'm reading the paper one day and the headline says "Boy in Texas Discovers Rare Disease with New Test". So this is when the testing called whole exome sequencing started. They basically look at every gene in the genome. So I found the geneticist in New York who was doing this and we were tested - myself, my husband, and Josie - and I remember it clearly, I remember the office walls closing in on me. We were told that Josie has something called FOXG1 Syndrome. But they also called it congenital Rett Syndrome, because that's what it was called before the NIH - the National Institute of Health in the US - gave it its own proper diagnosis name. I was told by that geneticist at the time that Josie will never talk; she will never walk; she will probably never sit up. When I asked the most dreaded question of "what is the lifespan", he looked at me and said, "teens at best". But there were so few children at the time diagnosed with FOXG1 syndrome - in fact Josie was the 60th person in the world - so the prognosis he gave me I have since learned does not have to be the case. And I also did decide at that moment: how could he know this? At the time, there were no clinical trials, there was no research underway. And about a week after we got Josie's diagnosis, that's when the seizures started. They started like a tidal wave. We couldn't sleep at night because we feared she could have a seizure in her sleep. For about six months, my husband and I alternated staying up all night watching her.
Phil - Josie’s still around. She lives her life as best she can.
Nicole - She is fed and gets her medicine with a G-tube. She gets a bevy of medicine at five in the morning, at six thirty in the morning. And then if she's well enough to go to school, she gets up and we get her dressed and get her ready and get her in her wheelchair, and her bus comes, and she's got a nurse who goes on the bus with her, and she will spend a full day at her special needs school. And she'll be asleep usually by six o'clock - she's exhausted - and then the medicines throughout the night.
Phil - What's it like to be the parent of a kid with FOXG1?
Nicole - It's so amazing to me that Josie cannot purposely wipe her nose if it itches. She can't eat pizza. She can't yell at me over the clothes that she wants to wear, which was such a joy of growing up that I had with my mom. And yet she smiles and laughs all day long. I don't want to underplay how hard it is; you know, being a FOXG1 parents is very hard. There are only now 650 children in the world known with FOXG1 Syndrome. So don't forget, Josie was number 60 in 2014; we're in 2020 and there are 650. We have helped put FOXG1 Syndrome on the map. We had it added to the epilepsy panel. So now when someone goes for genetic testing like I did with Josie, they will automatically be tested for FOXG1. We also got it added to the microcephaly panel, which is having a small head.
Phil - FOXG1 Syndrome has likely been around for the beginning of time - but nowadays, doctors know to look for it. Which means that, as with conditions like autism before, the official numbers are shooting up. What you miss if you just look at the numbers is that for people like Nicole - that is good news.

19:60 - FOXG1 treatment search: "coincidence after coincidence"
FOXG1 treatment search: "coincidence after coincidence"
Jae Lee, University of Buffalo
The FOXG1 Research Foundation in the USA has to date distributed a million and a half in funding dollars. The bulk has gone into building mouse models of the disease, led by scientists Soo and Jae Lee. They also happen to also be married, with a daughter who has FOXG1 syndrome. It's interesting but not that surprising - except that that they were neurodevelopmental experts long before their daughter was born. Jae Lee told the story to Phil Sansom...
Jae - I was also studying gene transcription, although in a different context, so when our daughter arrived and then the issue was FOXG1 syndrome... complete coincidence. I mean, we were kind of like really shocked. Another coincidence was that FOXG1 was one of the markers that my wife has been using in her studies, so she was extremely familiar with what the protein was. Kind of like coincidence after coincidence, right? We kind of took it as fate.
Phil - For both of them, it was a natural transition to just start specifically researching FOXG1.
Jae - Almost nothing is known about how FOXG1 functions, so that's why we are creating many mouse models.
Phil - These different models have different types of mutations in the FOXG1 gene. And these mimic the types of mutations you see in humans.
Jae - The spectrum of symptoms are very different because FOXG1 has different domains, functional domains, and some of them are way more important than others. For instance, the domain that's essential for DNA binding; when FOXG1 syndrome occurs through mutations in this DNA binding, that's going to be really severe symptoms. On the other hand, if you have a mutation in a non-essential domain, the symptoms tend to be milder.
Phil - This is what you see in kids with FOXG1 Syndrome - for example, Jae’s daughter Yuna has milder symptoms than Nicole’s daughter Josie.
Jae - This is a neurodevelopmental disorder, meaning that when children are born, the trouble is already there and you can not go back. And in principle, once the brain is built improperly, it's already done. In the case of FOXG1 syndrome, what we found was that the FOXG1 protein continues to be expressed in adult stages; and FOXG1 protein expressed in adult stages still playing really, really important roles.
Phil - For Jae this was big news. Not only does FOXG1 do its thing in the womb, it keeps on doing its thing. Meaning that fixing a problem will still help. In the case of the mice, if FOXG1 breaks after the little pups are born, their brains still get that characteristic shrunkenness.
Jae - That actually gave us great hope.
Phil - It sounds like you're talking about not just trying to stop FOXG1 syndrome ever occurring, but also trying to look for a cure for kids who already have it.
Jae - Absolutely, absolutely. It's not going to be a complete cure, but I believe that some of the symptoms can be treated. That would make the life of parents much easier.
Phil - It’s not usual to find a scientist with this amount of skin in the game. Jae is trying to find a treatment that will fix FOXG1 in the mice. He’s essentially working on subbing in a spare copy of the gene that won’t sit in the mice’s normal DNA, but instead will hang around inside a modified virus - what’s called a viral vector.
Jae - FOXG1 Syndrome is basically, instead of 100% of FOXG1, you only make 50%, right? So you can actually, using a viral vector that contains the FOXG1 gene - that can make FOXG1 protein - you deliver this viral vector to the patients. So you are trying to recover or restore the normal level of FOXG1 protein. We actually are at exactly at that stage right now. So now we can test whether any of those symptoms can be alleviated. So we are actually at a pretty exciting time to see whether we can do something.
Related Content
- Previous What else do mosquitoes eat?
- Next Colour on the Brain - part 1










Comments
Add a comment