Our world - and our bodies - are teeming with bacteria. And although some of them are friendly, many of them are not. Plus, electrifying news about bacterial nanowires, cuddly koalas, and counting chromosomes, and our gene of the month is the mindblowing Mind Bomb.
In this episode
01:03 - Bugs and bladder infections
Bugs and bladder infections
with Dr Jenny Rohn, University College London
Bacterial infections cause millions of deaths around the world every year. But countless more aren't fatal yet cause untold misery and pain. Urinary tract infections, like cystitis. are caused by bacteria getting into the bladder. They're very common - around half of all women are likely to have one at some point in their life - and as well as causing pain and discomfort, recurring or chronic infections can also lead to incontinence, especially in the elderly. But the reason for these recurring infections sounds more like a horror story than a scientific research paper.
To find out more, Kat Arney spoke to Dr Jenny Rohn at University College London - and the first thing I wanted to know was what happens when bacteria get into the bladder...
Jenny - Well, typically, they get up there and it's a nice, warm, wet environment and they're quite happy, but it's a rather hostile place because, of course, you are voiding your bladder quite regularly. So, if you're a tiny, tiny bacteria, say, you are 2 or 3 microns wide, and you're being faced by somebody peeing, it's like the force of the fire hose. Normally, this is what happens. The bacteria get up there and then you have a pee, and then swish, they're whizzed out and it's not a problem. But bacteria are very, very sticky. They've got special proteins on them that like to stick to cells, and E. coli in particular - which is one of the major causes of UTI - is really, really good at sticking to cells.
OK, so normally, this is what happens. The cells of the bladder, they're sort of there and the bacteria get up there and they manage to evade the peeing process. And they're clinging on there, they're sticking on, and they're causing a little bit of havoc and mayhem. This is when you might actually start feeling some symptoms. You might feel some burning when you pee. You might start peeing very, very frequently and then you might have a look at your pee, and you might notice it's really cloudy, and maybe there's some blood in there, and you know you've got a bad infection.
And then you go to your GP and your GP gives you antibiotics, and you take them for a week. You feel better usually. End of story, right? But actually, it's not the end of the story. You might get it again in a few months and many women suffer with these recurring UTIs. It doesn't make any sense because this women are usually not having bad hygiene. They can't shake this bug.
Kat - So, what's going on there? What's making this certain bacteria just hang around?
Jenny - The key to the mystery was solved about 10 years ago and this observation that bacteria are not always just sticking to the outside. So, a bunch of scientists worked out the bacteria are brewing inside the bladder. So this is very, very strange. E. coli is a free-living organism. It's not a parasite. It's never, never supposed to go inside cells and in fact, the cell never lets stuff go in there that's not supposed to go in. So if you were a cell, you got invaded by a cheeky bacteria, you'd throw up a big cage around it and you degrade it, and get rid of it. This is what happens all the time when cells are molested by various things. They just shut them down. Also, the immune system should be doing this stuff and getting rid of these things.
What happens is when the bacteria get inside the cell, for some reason that we still did not understand, they just escape. They escape all of these controls. They're probably in some sort of bubble, so they've been internalised by the cell and they're in some sort of bubble, and this bubble is supposed to be heading toward the degradation machinery, the stuff that's supposed to break them into little pieces and kill them. But for some reason, they escape from these bubbles. They get out. They have some sort of communication with the molecules around and they manage to slip the net, they sort of breakout of these little prisons. They must be tricking the cells somehow because a cell shouldn't have to do that. So we think there's some sort of weird molecular communication going around that's fooling the cell into thinking that this bacteria are supposed to be there and they're supposed to be released.
So once they're released, it all goes really, really strange. So they start dividing, and they start dividing, and dividing and dividing. It's like a big mass of bacteria inside the cell and they get bigger and bigger, and bigger. Meanwhile, you don't know you're sick. You feel fine. You've got...
Kat - No sign of any of this.
Jenny - No sign of anything, but deep inside your bladder, there's these hidden colonies of bacteria that are growing and growing. And then actually, if you look at them down an electron microscope, you can see they're actually pushing at the cells. If a cell is a like a balloon, it's this huge mass inside that's pushing the cells out, distending it. You can see these big bubbles which are known as pods which you think is great, but...
Kat:: This sounds like some kind of horror fil!
Jenny - Yes, it's so alien because you know what's going to happen next, right?
Kat - Out they go and they all start again.
Jenny - At some point, the pod get so big that cell literally explodes. And these guys just whizz out. There's millions of them, billions of them. They whizz out into the bladder and start all over again.
Kat - What's going on then at a molecular level? Do you have any clues about some of the genes and the proteins that are actually involved in this weirdly subversive process?
Jenny - Hardly anything, I'd say. There's been a few studies done suggesting that there's a protein called actin which is inside all our cells that makes a bit of a skeleton and it keeps the cell a certain shape and everybody's got actin. And there's a thought that maybe in these bladder cells, the actin is supposed to be keeping it in check and it gets somehow subverted, but really, nothing's known. Absolutely, not. That's why I'm so excited about this project because it's completely a wide open field.
Kat - So then, tell me about how you and your lab are trying to approach this question?
Jenny - We've got two basic approaches on our lab. One approach deals with how we're going to treat these people, how can we design better therapies? Because the pods are inside the cells, the antibiotics that you take can't get in there. So, antibiotics, many of them cannot pass through the cell membrane. So, we need to find better ways to get antibiotics inside the cells. And the other thing is just basic cell biology. What happens when the bacteria get inside? What does a cell do? What genes are required to allow these bacteria to escape from these cages? There must be something going on, so we'll be using a genetic approach, whereby we knockout genes that we think might be involved and see whether that stops the bacteria from getting out. So, it's the sort of basic approach where, if you wanted to know how a car works, you could systematically break each part of the car and see what happened. It's the same sort of thing. We're going to systematically interfere with genes that we think might be involved and then see whether that affects the infection process.
Kat - So how many genes are you going to start looking at?
Jenny - Well, there's about 20,000 genes. We've got a collection now of maybe 50 genes that I think might be implicated. They're genes that are expressing proteins that are near the surface of the cell, genes that express proteins that tend to be involved in trafficking of particles to and from the surface. So there are sort of things that are around there anyway, things that will probably be hanging out when these bacteria dive into the cells. And there's a number of leads we have and I think that we should be able to find something. I also want to do some really interesting live imaging where we can make green bacteria and infect cells and see if we can actually see the bacteria going in and what they're doing.
Kat - And then presumably, watch them exploding back out.
Jenny - Yeah, there are - I've seen some amazing movies so I think that is possible. This technology makes it possible and I think it will be quite fun just to watch, but ultimately, with cell biology, you can't learn everything just by looking. Things are so microscopic and complicated that you have to start doing sort of genetic interference to see things.
Kat - What could be the benefit if we could make significant improvements to understanding and treating these UTIs?
Jenny - I think that if we understand how the bacteria get in, how they persist, and we should be able to prevent that from happening in the first place. I mean, you will never be able to prevent cystitis. Women will always get urinary tract infections, but what we will be able to prevent, if we know how it works, we can prevent the sort of invasion and the long term colonisation of this sort of alien pod-like thing. If we can prevent that from happening by targeting it with the drug, that would be brilliant. We see 150 patients a week in the clinic here and these people are really, really miserable and I think that if we can make a difference by studying this bacteria and improving their therapy, it will make a huge difference to their lives.
09:26 - Genes and the placebo effect
Genes and the placebo effect
with Nell Barrie
Nell:: The first story we're looking at is looking at the placebo effect which is this idea that patients can get a benefit even when they're not actually being treated with anything. And they're looking at the genes that could possibly contribute to this. So, this is published in PLoS ONE and the researchers have looked at a particular gene that has a role in regulating the dopamine levels in the brain. So this is to do with our kind of reward and pleasure responses to things.
Kat:: It's like the happy chemical, isn't it? It makes you feel good, all that kind of stuff.
Nell:: Exactly and the idea, their sort of hypothesis at the beginning was that perhaps people who can produce more dopamine in their brain, so they can have more of this sort of pleasure response to specific things, maybe they could be more susceptible to the placebo effect, and they wanted to test this. So they did that by getting some patients who have IBS, irritable bowel syndrome, and looking at different types of treatment. So, that ranged from them being on a waiting list and getting no treatment at all, or they could have a placebo acupuncture which is an interesting concept.
Kat:: I wonder how that works. They just stick them or something?
Nell: Yeah, it's a sham acupuncture device. So probably, you can feel it, but they're not actually going through the acupuncture procedures or they got the acupuncture placebo treatment so fake acupuncture, plus to getting talked to really nice, friendly, supportive doctor who listen to their concerns. And they compared all those different things and found that people with this genetic change that meant they could produce more dopamine, responded a lot better to the supportive doctor and the placebo acupuncture, so it's quite interesting.
Kat:: I think this is fascinating because it's interesting that they chose IBS which is a condition that it's quite chronic, it's difficult to know what causes it, it's difficult to know how you treat it. And it seems to me that many of the conditions that respond to alternative treatments, complementary treatments that are considered by many to be placebos are these kind of very chronic diseases. I wonder if it would work for other things, just people would be more susceptible to having a nice hot bath or going shopping?
Nell:: It is really interesting because the placebo effect is really powerful and it really can help make people feel a lot better even when we're looking at really serious diseases. So, if we can find a way to sort of harness that, figure out who could benefit most, how can we use the placebo effect to our own ends. That would really be exciting. It's a small study, but it's sort of pointing us in a direction of you know, what's happening in the brain, how could this affect how you respond to the placebo effect.
12:08 - Bacterial nanowires
Bacterial nanowires
with Nell Barrie
Kat:: Now I saw a paper this month in Nature which is from a team in Denmark in the US and this is electric bacteria. I absolutely love this. It turns out that there's little bacteria, tiny, tiny bacteria, which you think are solitary beings and they gang up together and make tiny electrical wires. Why are they doing this?
Nell:: So, these are bacteria that are living in that really kind of stinky horrible mud you get if you have a go at sort of walking by the seaside. You're in your wellies and you stump into the mud and it smells like farts, and it's just utterly gross. But the bacteria living in there and part of the reason why the mud smells so foul is because there's no oxygen in there to help break down all the kind of gunk and stuff. The bacteria have a problem with this too. If they're living really deep down in the mud, they can't get the oxygen which is up at the surface. So, they are teaming up. They're forming these little filaments right through the mud, so that the bacteria at the top can put electrons onto oxygen and the bacteria at the bottom are taking electrons off sulphides in the mud. So, it's like a kind of team effort to breath and eat at the same time, but doing it altogether.
Kat:: It's really fascinating that you think of bacteria, just as being solitary things, but actually, there's quite a lot of single-celled organisms that gang up together and do things like this and this is really interesting example. I wonder if we you could make living microchips or living electrical devices with these bacteria.
Nell:: There's always been this idea that multicellular organisms might have evolved in this way perhaps. So you start off with your one bacteria. They start teaming up together and when it gets to the point that they can't work without each other any more, essentially, you got a whole new organism. So, it's quite interesting to see something that's sort of in between the two extremes if you like.

13:50 - Koalas in trouble
Koalas in trouble
with Nell Barrie
Kat - And speaking of things evolving, there's some worrying news about koalas I saw this month. This is very sad news. What's going on here?
Nell - So, this is something we're hearing more and more about which is a bit of a shame. So koalas obviously are not doing particularly well in the wild because of various things. They've been hunted, they've had problems with disease, and this were some researchers going back to see whether old koala bones, examples that we have in museums for example, whether they had more genetic diversity than koalas today, and their idea was that, back when there were many more koalas around, there'd be a lot more diversity in the genes that all the animals had. And what they found is that actually, koalas have had quite low genetic diversity for over a century, over 120 years. And it is a real shame because it's meaning that it's getting harder and harder for them to cope with what the environment is throwing at them, things like Chlamydia for example. It sounds really weird, but koalas are actually really badly affected by Chlamydia.
Kat - Really? That sounds so sad.
Nell - I've found out about this when I was in Australia because I just went to look at all the cool animals. We went to a place called Kangaroo Island which is a big island right at the bottom of Australia where they've got a great wildlife sanctuary set up. It was really nice to be there, but we were looking at a koala and this guy just saying, "Oh, it's really great because the koalas here don't have chlamydia." And I was just standing there going, "What?"
Kat - Eww!
Nell - Koalas with sexually transmitted diseases, it's really bizarre. But actually, loads of koalas on the mainland have chlamydia and it kills them. We think that perhaps the fact that they've got this low genetic diversity means that there's not much resistance in the population and they're not doing very well at sort of evolving to cope with this.
Kat - It's sad that you think the populations, when they get so small, there really isn't a lot of hope for them. I hope there is hope for koalas.
Nelly - Yeah, exactly because I mean, I guess the question would be, where do you get extra genetic diversity from? Once it's gone, it's gone which is a real shame. So I mean hopefully, we can find ways to cope with this and I guess the main thing for researchers would be, this is a lesson for how you deal with animals that could get to that point. We have to stop that from happening.
Kat - Well, let's keep our fingers crossed for the koala bears. Thanks very much, Nell.
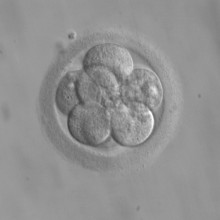
15:54 - Three-parent embryos created
Three-parent embryos created
Scientists in the US have made medical history by creating human embryos with three parents. This is a step towards using IVF techniques to solve so-called mitochondiral diseases, caused by faulty 'power stations', or mitochondria, in a mother's egg cells. Because an embryo's mitochondria are only provided by the egg cell, researchers think that transferring the nucleus from an egg with faulty mitochondria into a donor egg with functional ones could sidestep the disease and create healthy babies. Writing in the journal Nature, Dr Shoukrat Mitalipov and his colleagues took the nucleus, containing DNA, out of human egg cells and transferred them into egg cells that had had their nucleus removed. These composite eggs were then fertilised by human sperm, and allowed to start developing.
This technique has already been successfully used in monkeys, leading to the birth of healthy monkey babies, and these new results show that it's potentially feasible in humans, although the embryos were destroyed after only a few days, when they were just a small cluster of cells. The UK recently launched a consultation on whether to consider the technique for treating patients with mitochondrial diseases, and this new study answers some questions about whether the technique could actually work in huma
17:14 - Gene linked to old-age hearing loss
Gene linked to old-age hearing loss
Researchers at the University of South Florida have tracked down a genetic change linked to old-age hearing loss - something that affects many millions of people around the world every year - publishing their findings in the journal Hearing Research. Thanks to a 9-year-long study of nearly 700 people, the scientists discovered that a certain variationS in a gene called GRM7, short for glutamate receptor metabotropic 7, were linked to a higher risk of hearing loss later in life - the first time a gene has been linked to old-age hearing loss. GRM7 helps to convert the sounds we hear into nerve signals that go to the brain, where they're decoded and interpreted.
The researchers suggest that it might be possible one day to test people for the presence of the risky gene variations, so they could choose to take extra precautions to protect their hearing if they wished.

18:04 - DNA smart-gel
DNA smart-gel
It sounds like the stuff of science fiction, but Omar Saleh and Deborah Fygenson at the University of California Santa Barbara have developed a DNA-based "smart gel" that can contract and move in response to stimuli in a similar way to living cells. Writing in the journal PNAS, the scientists created a cell-sized blob of goo made of a mixture of short and long DNA strands, together with special proteins known as motor proteins, which can reel in or spool out the strands. By feeding the motor proteins ATP molecules, which act as an energy source, they move the DNA strands around, changing the shape and stiffness of the gel.
Because DNA molecules can be engineered to have different properties by changing the sequence of 'letters' in them, and because there is a huge range of motor proteins that could be used, this discovery potentially opens the door to bioengineered 'smart materials' that could be used to make artificial muscles or other useful things. The researchers are now refining their blob, to try and create something that can move in specific ways, such as twisting or crawling, which would bring more control.

19:29 - Germ genomes
Germ genomes
with Dr Matt Holden, Wellcome Trust Sanger Institute
Kat:: But now, it's time to take a closer look at bacterial genes. I spoke to Dr Matt Holden at the Sanger Institute in Cambridge to find out how advances in genome sequencing are helping researchers and doctors to understand more about bad bacteria, and even to track infections as they happen.
Matt:: Genome sequencing over the last say, 15 years of about bacterial sequencing has been very informative about having this amount of contents of a genome, and it's really propelled research forward. It allowed biologists and geneticists to have a glimpse of what the genetic blue prints of many important pathogens are and from that, try and dissect what are the important genes, what are the genes that allow it to survive in certain niches, what are the genes that maybe cause a damage when they're causing disease. In this case, we're now moving to a situation with sequencing where the technological advances of sequencing mean that rather than being a fundamental sort of research tool, which is sort of based in institutions like the Sanger Institute where I work and university labs, it's now been sort of transited to the sort of setting of a diagnostic lab because of the reduction in cost and increase in throughput.
So, we're now looking at genome sequencing as a diagnostic tool, so when you are identifying a bacteria, you won't just identify them by maybe culturing them on a selected media. You'll actually sequence the genome. So, for a pathogen that's been isolated from a disease situation, you can identify or reconstruct the genome and identify what genes it's carrying which can be very important when you're trying to provide information about what antibiotics to use because from the genome, you can predict what antibiotics the pathogen may be sensitive for. The key to this is, it can be done very cheaply or is certainly becoming cheaper and also, it can be done rapidly which means it can certainly provide clinicians and those involved in the management of disease, important information within a very short space of time.
Kat:: One of the things that you did recently was to actually track an infection, an MRSA infection. How did you go about doing that and what did you find?
Matt:: What we wanted to do was to use genome sequencing to look at the isolates that were identified in this outbreak that occurred on a neonatal intensive care ward and to see what information genome sequencing will provide that would help combat this sort of transmission in the future. So, we were also interested to discover what it told us about the outbreak itself or perhaps standard clinical diagnostics and infection control didn't. So we sequenced the genomes of 14 isolates that were identified as outbreak and also, additional isolates of MRSA that were identified in the hospital at the same time. And showed through genome sequencing, that we were able to effectively reconstruct a family tree for these bacteria.
So, what we were able to do was identify very clearly which bacteria are involved in the outbreak and showed that these were very distinct from the other bacteria that were floating around in the hospital at that time. But we're also were able to go into the genomes and predict what antibiotics the microorganisms were sensitive to. So, providing information that clinicians could potentially use very quickly to treat patients who are becoming infected and also, predict which toxins they were carrying which again, can be important to inform clinicians as to how to manage disease when it occurs in a clinical setting where you have potentially pathogenic bacteria that have got important toxins involved that can cause particular types of disease.
Kat:: How do you think being able to track the genetics of bacteria in this way will be useful to doctors and beneficial to patients in the future?
Matt:: Well obviously, the study we've done recently is very much a sort of proof of principle and so, it's a case where we're seeing what the potential of it is. Obviously, with technology changes, with technology progress, the cost of sequencing will go down and also, the rapidity with which you can sequence the genome is going to go up. So, the immediate benefit will be hopefully that you will be able to identify what bacteria is associated with infection, and whether or not it's related to other bacteria that part of an outbreak very rapidly, hopefully quicker than standard techniques. And also, with the decreasing cost, you can do it more cheaply.
The other benefit is you have the entire genome sequence there, so you can actually look at all the contents and provide all this additional information that we currently don't have. We get these extra resolution and be able to distinguish related bacteria, but we also get all the genetic information and it gives us a new insight to what the bacteria are carrying.
Kat:: That was Dr Matt Holden from the Wellcome Trust Sanger Institute.
What determines the chromosome count of orga?
Answered by Dr John Welch, Department of Genets, University of Cambridge.
John:: Well, there are two quite different, but perhaps equally important why so much chromosome number can change. But first, sometimes there's a problem when the cell makes copies of itself and that ends in the complete genome doubling, we end up with two complete copies of the genome. This does seem to be an important process in the evolution. It has contributed to change in animals, in fungi, in single-celled protozoa, but it's hugely important in the evolution of plants. The second important way in which chromosome number can change is by two chromosomes fusing into one or one chromosome splitting apart into two. This is something that's happened in our own lineage for example, our own chromosome Two seems to be the result of the fusion between two chromosomes which were present in our most recent common ancestor with chimps and bonobos. The important thing about this type of change in chromosome number is on the whole genetic information that's present in the cell doesn't change very much. And this means that in this case, for the person with the unusual number of chromosomes or the organisms with an unusual number of chromosomes, there doesn't seem to be many consequences at all of changing the way that that information is distributed amongst chromosomes. But then there can be problems for the fertility for that individual when they go on to try and make sperm or eggs, in other words, becoming increasingly clear from experiments is that the reduction in fertility, the partial sterility seems to be much less than we predict. It seems to be only a small 4 or 5 per cent for example, reduction in fertility in individuals with a small number of chromosomes. This means that while the change in chromosome number might be subject, might be selected against natural selection might have to slightly reduce the number of odd numbered chromosomes in the population. This effect can be rather weak and so, just random sampling effects taking place over the generations can mean that the abnormal number of chromosomes reaches a high frequency in the population without weak natural selection being able to counteract this effect, and that's probably an important mechanism by which chromosome number changes in practice.

27:48 - Gene of the month - Mind Bomb
Gene of the month - Mind Bomb
with Kat Arney, Naked Scientists
And finally, our gene of the month is Mind Bomb. First described in zebrafish, fish with faults in their Mind Bomb gene have big problems with developing a nervous system or muscles. And in their ears - yes, fish do have them - they have ten times as many sensory hair cells as would be expected, these are the cells responsible for picking up vibrations around them. They also twice as many nerve cells in their ears as normal fish. In 1998, Dr Julian Lewis and his team at the London Research Institute figured out that Mind Bomb must be playing an important role in the Notch signalling pathway, which helps to tell cells what fate to adopt, or what job to do.
As you might expect, there are versions of Mind Bomb in a host of other organisms, including mammals, where it's involved in making sure the brain and nervous system develop properly. Earlier this year, scientists showed that Mind Bomb also plays a role in the adult mouse brain, and is essential for helping to create long-term memories. Mind Bomb is also the name of a pretty lethal cocktail made with pure alcohol of the kind you would find in a molecular biology lab. Don't try this one at home!
- Previous Ricin: Chemistry in its element
- Next What is Ash Dieback?










Comments
Add a comment