Every biology student is familiar with DNA - the ladder-like blueprint of life built on a backbone of the sugar deoxyribose. Scientists are now hacking this structure to make entirely new DNA-like molecules built on different sugar skeletons, opening an exciting new world of synthetic genetics. Plus, we find out what happens when music has sex, discover why the X chromosome is more than just a number, and our gene of the month is the unfortunate Ken and Barbie.
In this episode
01:10 - Synthetic DNA - Dr Phil Holliger
Synthetic DNA - Dr Phil Holliger
with Dr Phil Holliger, MRC Laboratory of Molecular Biology
Phil:: We're trying to change the type of media, if you want in which one could write it. If you think of the Rosetta stone where you have the same message rendered in three different scripts, it's the same - the message is the same. It's just the writing that's different and really, that's what we're trying to do, by building different scaffolds on which the letters of the code can be mounted on, if you want.
So we replace the ribofuranose with a whole range of different chemical structure - 6-membered rings, 5-membered rings, interlocked rings, and we've been able to show that these alternative scaffolds retain the ability to display the code in a readable form. So really, that basic function of storing and propagating genetic information is not unique to DNA and RNA, and that is a profound result that tells us a lot about - I guess both the chemical basis of life on earth and potentially, elsewhere in the universe.
Kat:: So life for millions of years on this planet has been built on this structure of deoxyribose, DNA, sugars, and RNA, and you're saying that you can make basically, ladders of information based on different sugars. Is there any particular reasons that evolution selected DNA and RNA, and didn't select these other sugars?
Phil:: We obviously have not tested out these other scaffolds to quite the same degree as life has tested out DNA and RNA, but what one could say with some certainty now is that the very basic functions of encoding, storing, and propagating genetic information and not unique to DNA and RNA. And I think the sort of inescapable conclusion is that life chose DNA and RNA, not for fundamental functional reasons, but presumably, you know, for opportunistic reasons. So, they represent, if you want, a frozen accident from the origin of life. RNA at that time was around, did the job and that's what life got started with. Once you've made that choice, you know, you stick with it. There's no reason to change.
Kat:: It's a bit like VHS and Betamax or Blu-ray and HD DVD - people just go with one and that becomes the main one.
Phil:: Yeah, to some degree. Obviously, the current thinking is that life really got started on RNA and as the genomes grew bigger, RNA was slightly too unstable for the job. So, life moved on to DNA for storing information but retained RNA for many of its other functions. So that's really how far life got and there was never any need to go any further than that, but it could have started potentially on some other backbone.
Kat:: What do you think are some of the applications for these new types of sugar-based codes that you're developing?
Phil:: There's a class of single-stranded nucleic drugs made from DNA and RNA called aptamers and really, these have the potential to rival things like antibodies at least in some clinical settings. But I think one of the reasons that really haven't had such success in the clinic so far is that the DNA and RNA are very much degradable and degraded by the body.
So, they generally need to be modified to have a chance to work as therapeutics and that really drives up the cost, lengthens the development process and makes them in some cases also compromises their actual functionality. So, one of the advantages of these new backbones is that they're very, very hardy. I mean, some of the ones we've worked with, they're essentially - can't be degraded in the body and they're also very, very chemically inert, for example, very stable to sort of acidic conditions that you might encounter in the stomach. The hope is that we can build much more resilient therapeutics and potentially, also diagnostics and other structures from these backbones that will have a whole range of utilities.
Kat:: But if these - these are basically are life codes and they can't be degraded, they can't be broken down. Are there risks of putting something like that out in the environment?
Phil:: Well, if I said it can't be degraded, I think they can't be degraded within the sort of useful lifespan that you would think of a therapeutic. I think everything would eventually be degraded and excreted by the body, but you know, rather than being degraded within an hour of entering the bloodstream, these, I would expect them to last for days, weeks, possibly months.
Kat:: So not some kind of rogue code that's going to take over your body.
Phil:: As I've said, there's nothing rogue about it. I mean, it's really, the code has not been changed. It is really just the scaffolding that has been modified.

07:08 - Evolution of music
Evolution of music
with Nell Barrie
Nell:: First one that we thought was exciting was a really interesting study looking at the evolution of music and this was published in the PNAS and it was led by Robert MacCallum and Armand LeRoi from Imperial College and what they've done is essentially, they've got some random sections of - I guess you wouldn't even call it music - just notes, random notes put together.
And they're getting people to select them on the basis of what they like to hear, what sounds the most pleasant and then they're getting these little selected bits of music to mate together and make babies, and make new music by just randomly combining bits in the tune and they're going to see if that can lead to music evolving over time which is very interesting.
Kat:: So, we can listen to a bit of this. So, we've got some of the tracks, the Darwin tunes as they're called. So here's the first track - Generation Zero. So, these are just randomly generated noises... And here, we have them after about 400 generations...And now, here after about 1,200 generations, quite like the sound of this one...And finally, after about 5,500 generations, so these are really quite long and developed now... Do you think this is really natural selection and evolution though?
Nell:: I think it's really interesting model, I guess, but it's not really the way that natural selection and evolution works with animals and organisms because you're basing the selection on one very specific criteria which is whether the particular person listening to it actually likes it and in reality, if you're looking at an animal in its environment, there's all kinds of other things that will act to select whether it fits that environment or not I suppose. And also, this isn't really over a long period of time because you normally look at how the environment changes over time, what does that do to the organisms within it.
Kat:: Exactly, it's a very split second thing - do I like this? Do I not? - I think quite interestingly, they're all very major, quite bland really. I think if this is the future of music, I'm not sure about this.
Nell:: No, it definitely sounds kind of like something out of Pacman maybe and I mean, I like it from that point of view and it does sound like real music I suppose, but it's not - yeah, it's not going to set the world on fire any time soon.

09:51 - Blocking pathological rage - in mice
Blocking pathological rage - in mice
with Nell Barrie
Kat:: Something that may - well, set something on fire is a story that I saw in the journal of Neuroscience. This is from Marco Bortolato at the University of Southern California and this is looking at the genetics of rage. This is an absolutely fascinating story. What are the researchers been up to here?
Nell:: Yeah, I really like this one because I can definitely identify with extreme rage at some points in my life.
Kat:: You look so calm and peaceful all the time.
Nell:: No, I definitely have that feeling occasionally where you just kind of snap and you get really mad. And perhaps, this is telling us a little bit about what might be happening in the brain especially in people, perhaps you have a real problem with controlling that rage and just can't manage to control it at all. By looking at a specific receptor which they think might be malfunctioning and over functioning perhaps in people who are very hostile, and they've looked in mice. So, this is really early stage stuff, but they found this receptor is faulty in mice that are very, very hostile and they think that this could have something to do with the same process in humans perhaps. So, maybe there could be a way we could fix the function of that receptor, return it to normal, and could that help us treat people who have rage problems maybe?
Kat:: You know, we're not talking about people who like you, just get a bit of a strop on...
Nell:: No, just getting a little angry is more like kind of serious problem with rage, isn't it? The other thing that struck me reading this is it's one of those studies where you're looking at a tiny part of what the brain does. And it's really interesting and it's telling you something but it really needs to be part of the whole thing, and when you're looking at things like behaviour, it's usually not simple as switching one thing on and off, and switching that behaviour on and off because there's so many different aspects to it, and they talk about risk factors as well.
So the way you're brought up, the kind of environment you live in when you were a child has a big effect on the type of rage response you might have to things. So, that really got to be taken into account as well, so it's really early days. But it's interesting to see how these things are similar in mice and humans I think.
Kat:: I'd like to see a mouse with rage - eek!!
Nell:: you can see videos of angry rats on the internet that I've seen. They've done some selective breeding and you get really, really angry rats who just attack people just on sight.
Kat:: More than angry birds who just attack pigs.

11:56 - Super-scissors for DNA
Super-scissors for DNA
with Nell Barrie
Kat:: The final story that we've got is a really nice paper published in Science. This is from Jennifer Doudna at the University of California Berkeley and I love this just because of the potential of the story, and this is researchers who've been looking at bacteria and looking at how bacteria use certain molecules like molecular scissors to snip up their DNA and glue it together, and it helps them develop different characteristics to help them survive. But they've actually looked at these molecular scissors and found out how they work. And they think that possibly, you could use this technology to make programmable scissors so you could cut up human DNA, animal DNA, and basically glue it back together in any kind of way you like.
Nell:: That's pretty cool. I mean, I think it's kind of what I think of as genetic engineering. I guess you think you'll just put together whichever bit of the DNA that you want, but actually, in reality, that's not really possible yet. So I suppose this is taking us a little bit closer to real designer genetic tailoring or whatever you might like to call it.
Kat:: So I remember when I was in the lab - and this is some time ago so things may have changed - but if you wanted to do genetic engineering and stick different bits of DNA together, you had to look at the sequence and there were only certain sequences that you could cut using enzymes. Things have probably moved on now, but I think there is some restriction in the sequences that you can cut and glue together. So having a different set of technology could really be exciting.
Nell:: Yeah, and I guess that sort of eventual implications would be that you could be create these kind of designer organisms like maybe, bacteria that degrades nasty environmental toxins, or produce things that we need for drugs, or all kinds of exciting applications. Because we know that bacteria and fungi have all these abilities and it's just about, could we harness them to do the things we want, I guess.
Kat:: Watch this space, I think because it is still very early days for that.
13:51 - Finding a new flu gene
Finding a new flu gene
An international team of scientists have discovered a new gene in the flu virus, despite it being 30 years since the flu genome was first decoded. Writing in the journal Science, the researchers found the gene by analysing patterns of genetic change in thousands of different flu strains, including the notorious 1918 Spanish flu.
The new gene, called PA-X, helps to control how the body responds to the virus by damping down the immune response to infection. The discovery is surprising not only because the flu virus has just 12 genes - so finding a new one is a big deal - but also that PA-X seems to reduce the impact of viral infection rather than enhance it.
The scientists think that their finding will help to shed light on why different strains of flu cause more or less severe infections, and could pave the way for future anti-viral treatments.
14:54 - Genetic cut-and-pasting gave us limbs
Genetic cut-and-pasting gave us limbs
New research led by Jordi Garcia-Fernández and Manuel Irimia at the University of Barcelona and published in the journal Nature Scientific Reports suggests that genetic "cut and pasting" could have been the driving factor behind the origin of vertebrate limbs.
The scientists compared the hedgehog gene in simple invertebrate fish-like creatures called amphioxus with the version in zebrafish, which are vertebrates because they have a bony spine.
The team discovered that an event called a chromosomal translocation, where two bits of DNA in the genome get accidentally cut and pasted next to each other, led to the vertebrate version of the hedgehog gene - Sonic Hedgehog - being switched on in a new part of the body, which eventually evolved to become limbs.

15:36 - New gene could lead to tastier tomatoes
New gene could lead to tastier tomatoes
Hot on the heels of last month's announcement of the full sequence of the tomato genome, plant researchers at the University of California, Davis, have discovered a genetic tweak that could make bland supermarket tomatoes taste more like classic heirloom varieties.
Publishing in the journal Science, Ann Powell and her team searched for transcription factors - proteins that switch genes on and off - that are involved in controlling quality and colour as tomatoes ripen.
The team focused their attention on tomatoes that were unusually dark green before ripening, and discovered that these plants carried a particular gene called GLK2.
This gene is involved in the development of chloroplasts - the tiny power stations inside plant cells that convert sunlight energy into food.
Tomatoes carrying the gene have higher levels of sugars and other naturally-occurring chemicals, making them tastier as well as healthier.
The researchers hope that one day their discovery could help to transform supermarket tomatoes - which have been bred for fast ripening and easy storage rather than flavour - into tastier toms.
16:46 - Hacking the cell's machinery - Dr Jason Chin
Hacking the cell's machinery - Dr Jason Chin
with Dr Jason Chin, MRC Laboratory of Molecular Biology
Jason:: We've been interested in making a new version of the translational machinery that allows you to put together, assemble new proteins composed of new amino acids. And the way we thought about doing this is really to think about building. If we think about the translational machinery that normally makes proteins as being like a factory that essentially assembles a series of beads onto a string, with the beads being amino acids and you want to put them onto the string of the necklace in a defined sequence.
What we've been able to do is to really think about building in the cell a new factory that allows us to build entirely new types of necklaces with new types of beads. And so, at the molecular level, that involves creating a new version of the ribosome itself and new aminoacyl-tRNA synthetases, and tRNAs that can recognise new amino acids and put those new amino acids onto tRNAs.
Kat:: This seems like quite a challenging thing to do. Some of the molecules you're talking about are quite small, but ribosomes are enormous. How do you go about rebuilding a ribosome?
Jason:: To give you an idea of the scale, I mean, these are macromolecules in the cell, so these are molecules that are on the nanometre scale so it's true that a ribosome is large relative to an atom or relative to a small molecule, but it's still small relative to a person or relative to a cell. And so, what you have to do to engineer these components of the cell is to really use the ability to engineer the genes that the cell uses to make those molecules. So in the case of creating a new version of the ribosome, what we've been able to do is to create versions of the genes that the host cell machinery then uses to assemble ribosomes, but to put in new versions of these genes, and then to use the cell itself to assemble new versions of the ribosome that we would like the cell to be able to build.
Kat:: So what could be some of the applications of these new proteins that you could make?
Jason:: So there's a variety of things that we're actively pursuing in terms of making new proteins on these new versions of the translation machinery. One broad class of problem we're interested in is being able to put new designer amino acids into proteins that don't naturally exist in biology but have different properties that allow us to report actually on what the protein that contains these amino acids is actually doing in the cell.
So for example, we've been able to make amino acids that we can put into proteins in the cell that when you shine light on them, they then actually form an irreversible chemical bond between the protein and any protein that that proteins in the neighbourhood of and that allows you to find out at the level of basic biology who they actually socialise with and talk to in the cell.
And that's important for understanding at a very basic level what proteins are doing in the cells and what their functions might actually be and how they'd change shape, and who they talk to as a function of the state of the cell.

20:40 - Experimental evolution - Dr Tiffany Taylor
Experimental evolution - Dr Tiffany Taylor
with Dr Tiffany Taylor, University of Reading
Tiffany:: I'm an evolutionary biologist and I'm interested in experimental evolution. So, what that allows you to do is it allows you to - I'm using microorganisms or fast-replicating organisms that allow you to follow evolutionary processes happening in real-time.
Kat:: Because normally, when you think about evolution, you think about millions of years, population, stuff takes a long time. Give us an example of how many generations you can get through and what sort of timescale?
Tiffany:: Well, that's the fantastic thing about bacteria is that they have a generation time approximately 20 minutes so you can get through vast amounts of generations very quickly and the population densities can reach huge scales in a very small space so you can keep large libraries of all sorts of mutants and you can grow them up. A great thing about it as well is that you can keep these mutants in suspended animation just by freezing them and then this allows you to compete evolved ancestral strains together and get those measures on like competition and fitness in different environments.
Kat:: Can't even imagine doing that with humans, so we dig up some Neanderthals and have a fight with them.
Tiffany:: Absolutely, yeah. So what you're doing is looking at survival of the fittest between like you say, fossils and highly evolved species and then letting them compete and fight, and seeing who wins.
Kat:: What are you particularly looking into?
Tiffany:: Well at the moment, I'm looking at the evolution of the novel genetic code. So what that means is that we're getting a bacterial cell, we're changing the code in some way and then seeing how the bacteria can evolve and adapt to this code over time.
Kat:: You're talking about changing the genetic code. Does this mean you're changing the DNA or something else?
Tiffany:: The way that the genetic code is read is in triplets. So, the genetic code is made up of different letters and each 3-letter codes code for different amino acid. So, for example, ATG is going to code for a specific amino acid. So if we change what that triplet code codes for, so it codes for a different amino acid, essentially, you're forcing the bacteria to read this genetic code differently.
Kat:: And so, you change it and then they have to figure out what on earth to do with it?
Tiffany:: Exactly. Then all we had to do is let them evolve over time just by transferring them to new environments and just then go back into the genome and have a look at what mutations have occurred to allow them to adapt to this new code.
Kat:: And you don't really think of bacteria as being much under environmental pressure. What sort of different environmental pressures do you put them under?
Tiffany:: Well in the case of this one, the only real environmental pressure we're putting them under is that we're linking this trait to the production of a certain enzyme which is going to be vital for their survival in the environment because it's going to have such a large fitness decrease associated with changing the code. You need to put a selective pressure which means it's going to maintain this new code within the bacteria.
Kat:: But basically, if they can't evolve away of getting over this, they're going to die pretty fast.
Tiffany:: Exactly, yeah. Bacteria are very good at overcoming problems. I mean, they've been here for about 3 billion years, so I think that they probably will find a way and it is a bit of an interesting project that we really don't know what we expect to see. But I would expect to see changes quite quickly and probably, quite inventive changes that we haven't thought of before.
Kat:: And you're looking at bacteria, you're making them go through multiple generations, and mutating them. Are they ever going to change into anything other than bacteria because when you look at the billions of years of life on earth, we've seen species gradually evolve into different things? Are they going to change into something that's not bacteria?
Tiffany:: It's not possible in the timescales that we're talking about. I mean, bacteria do replicate very quickly, but to see these sorts of changes in what we call the macro-evolutionary scale, to actually see them speciate, takes an incredibly long time and much longer than anyone's career.
Kat:: Certainly longer than your post-doc!
Tiffany:: Absolutely!
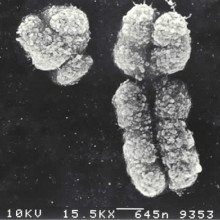
Why do we use the letter A,C,G, and T for DNA?
Louise:: With me this month is Dr. Robin Hesketh from the University of Cambridge. Hopefully, we have already answered that first part of that question, A, C, G, and T are for adenine, cytosine, guanine, and thymine - the four bases that make up DNA. But Dr. Hesketh, why are the chromosomes called X and Y?
Robin:: Let's just recap for a moment by thinking about human DNA and reminding ourselves that it's in fact divided into 46 different chromosomes. There are 22 pairs numbered 1 to 22, so the chromosome 1 is the biggest, with the most basepairs and chromosomes 22 and 21 are the shortest. And then in addition to them, there are of course the two sex chromosomes designated X and Y. In men for example, we have an X chromosome and a Y chromosome and ladies of course have 2 X chromosomes. Now the X chromosome was named following the general rule of algebra that the symbol X stands for the unknown quantity. X is nothing to do with shape. If you recall those chromosome pictures, most of them actually look like wiggly Xs, apart of course from the shrivelled Y chromosome, and its name just followed on from X.

Is it true that active genes make up only 3% of the DNA in our chromosomes? And if so, what does the other 97% do?
Robin:: Only about 1.5% of the human genome are regions that encode proteins and the rest of DNA, all the remaining 98.5%, is non-coding. Genes come in two sorts. The first sort are indeed those that direct the synthesis of protein and the second sort of gene are those that don't make a protein, but have some regulatory function as an RNA molecule. So these are non-coding RNAs and there's thousands of them in our genome. Now, all these genes whether protein coding or not, need to be controlled, switched on or off in the jargon, and that's done via non-coding DNA. The major controlling stretch is usually just before the start, just upstream of the gene itself and that's called a promoter. And then there are sequence motifs that are much more distant from the coding region and those are called enhancers. But even allowing for RNA molecules that don't make protein still leaves an awful lot of unemployed DNA. Some of that comes as telomeres. These are caps that sit on the end of each chromosome. They're repetitive stretches of DNA and essentially, they protect the end of the chromosome from deterioration or indeed from fusion with neighbouring chromosomes.
28:26 - Gene of the month - Ken and Barbie
Gene of the month - Ken and Barbie
with Kat Arney
Our gene of the month is Ken and Barbie - yes, named after the dolls. Like their plastic childhood counterparts, male and female flies with a fault in the gene have no external genitals - their naughty bits start to develop but get stuck inside the body.
The Ken and Barbie gene itself encodes the instructions to make a transcriptional repressor - a protein that prevents genes being switched on. Ken - as it's usually known - sits on genes involved in making a fly's genitals and keeps them switched off until the precise moment they're needed.
Luckily for us, there's no human equivalent of Ken and Barbie, although it's distantly related to a gene called Bcl6, which is involved in lymphoma.










Comments
Add a comment