Hearing loss is distressing, whether it occurs later in life or in childhood. Now researchers are starting to unpick the genetic causes behind some of these problems. Plus, mice on drugs, stress and death, and a wobbly gene of the month.
In this episode
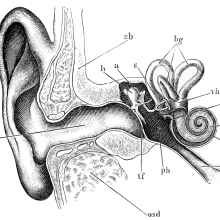
00:58 - Prof Karen Steel - Genes and deafness
Prof Karen Steel - Genes and deafness
with Prof Karen Steel, King's College London
Kat - Around one in six people in the UK has some kind of hearing loss, including most people over 70, and the number is rising. Professor Karen Steel, now at King's College London but formerly at the Wellcome Trust Sanger Institute, is an expert in hearing loss in old age, and has played a major role in tracking down some of the genes involved. I spoke to her about how far she and her team have come, and how they're trying to use this knowledge to help people at risk of losing their hearing.
Karen - We've identified a large number of genes, well over a hundred genes, that are involved in deafness either in humans or in animal models. We use the mouse mainly as our animal model. We know a lot of these genes are very different sorts of genes. They vary from genes called transcription factors which control the activity of other genes, or they could be structural proteins, they could encode structural proteins. There's a whole variety of them. When I started working on genetics of deafness, I thought that all genes involved in deafness were going to turn out to be ion channel genes. But of course, it wasn't - the first one that we identified was a gene called myosin VIIA, which is an unconventional myosin molecule.
Kat - That's a kind of "muscley" sort of protein.
Karen - That's exactly what you'd think. There's lots of other myosins in the body that perform other functions other than muscular contraction and this was one of them. This one was very important for both hearing and for vision, as it turned out. It turned out to be involved in a human disease called Usher syndrome where children are born deaf and with a balance problem. In the first 10 years or so of life, they start to develop visual problems as well until they lose their vision. So, it's a very nasty disease for the children to have and identifying several of the genes involved in that has been a very useful start to thinking about how it could be treated.
Kat - So, we have genes that are involved in hereditary deafness and hearing problems, and then there's presumably genetic variations between us as humans that mean maybe some people will lose their hearing earlier as they get older or struggle with different aspects. Do we know if it's the same kind of genes involved or different sort of genes involved in different types of hearing problems?
Karen - That's a good question. When you talk about hereditary deafness, I think most people are thinking about individual families where some children are born with a hearing impairment and others are born without a hearing impairment. That is very clearly genetic and usually a single gene that's affected that's causing that pattern of inheritance. But actually, genes do a lot more.
As you say, there are lots of genes out there that vary from individual to individual, and some of those genes can make us more or less likely to have damage to our hearing as a result of environmental problems. Some of them are important for the long term maintenance of the hearing structure in the ear. And so, as we get older, it may well be that just a single gene can make us more likely to lose our hearing. So, there's lots of different causes.
At the moment, we know quite a lot of genes, over a hundred in humans alone that are involved in childhood deafness that we know very, very little about the genetic basis of hearing impairment as we get older. And that's one of the main things that I moved here to Kings College to study because it's a basic neuroscience question - why is hearing gradually deteriorating as we get older? Is it a general part of growing old or is it something that's very specific to the auditory system? I think we'll the evidence is pointing towards it being very specific.
Kat - Not just because everything is falling apart?
Karen - Exactly, yes. So, there's lots of people who are very old who have perfect hearing and other people who relatively young who have very poor hearing. So, it's not just a general aspect of growing old. There are specific factors that make our hearing progressively get worse as we get older at different rates from individual to individual.
Kat - So, how are you trying to get the answers to this question?
Karen - I'm a geneticist, basically. So, I'm always thinking about how we can use genetics as a tool to get an understanding of the molecular basis of hearing and progressive deafness - particularly, progressive deafness. Because I think with progressive deafness then we have a much greater chance of being able to develop treatments. So, progressive hearing impairment is the main thing that I'm interested in. The technique I use is to look for single genes that when they're mutated or changed, will lead to progressive hearing loss. I use the mouse as my main model system because there are many models now or mouse mutants where they have a particular mutation in a particular gene that we've been able to show have progressing loss of their hearing. And so, we think, mimic the human progressive hearing loss.
Kat - How do you tell if a mouse is losing its hearing?
Karen - We use a physiological measurement called auditory brainstem response measurements or ABR. This is exactly the same technique that many hospitals use to screen newborn babies for deafness when they're born. So, it's a non-invasive technique and it's a very powerful technique to carry out at different ages that you could follow that progression of the hearing impairment in a mouse as it gets worse and worse. We can distinguish lots of different aspects of their response including whether you need to deliver a higher level of sound in order to elicit a response. And also, whether different frequencies - high frequencies or low frequencies - are responding at the right sort of threshold. This is quite important for humans because humans, as they get older, have a tendency to lose their sensitivity to high frequencies first. That's the earliest sign of progressive hearing loss.
And we found a number of different mouse mutants with single gene mutations that did have progressive hearing loss starting at the high frequencies. So, we think that these particular mouse mutants are going to be very useful to us in providing models for the normal natural history of progressive hearing loss in humans.
Kat - So, where would you like to see this research going maybe over the next 5 to 10 years, given the pace at which everything is accelerating in science and technology?
Karen - Well, we have some very good clues now. We have a number of different genes that we've identified as being involved in progressive hearing loss. Now, we're in a position where we can start thinking about what molecular pathways those genes are involved in and whether those are pathways that we can manipulate particularly, using small molecules. So, the traditional drug approach. This is an approach that really hasn't been very seriously adopted in hearing.
Most people think of hearing being treated by using hearing aids or perhaps cochlear implants if it's a very severe hearing impairment. Those are prosthetic devices. Those aren't treating the hearing because the person still has the hearing impairment. Whereas, we think that we should be able to develop some drugs or small molecules that will stop the progression of hearing impairment. But this is a long time off.
We think in the next few years - next 2 or 3 years, we should have some animal model work that will give some proof of principle of using small molecules to stop the progression of hearing impairment. Developing that to get into the clinic is going to take a lot longer as I'm sure you hear for many different diseases. It's always a long process to get the initial concept of using a small molecule to treat a disease from an animal model into the clinic. So, deafness is no different from that.
Kat - That was Professor Karen Steel from King's College London.
08:35 - Narcolepsy and flu
Narcolepsy and flu
with Nell Barrie
Kat - And now it's time to take a look at the latest genetics news with science writer Nell Barrie. So, the first story that I wanted to talk about was one about narcolepsy and flu. So, an interesting connection between randomly falling asleep, which is the disease narcolepsy, and the flu virus. What's this about?
Nell - Well, it seems that the link here is to do with the immune system which as always is very fascinating and very complicated. What these researchers have found, it's these people at Stanford University School of Medicine in the US and it's published in Science Translational Medicine. They've actually discovered that narcolepsy seems to be an autoimmune disease. So, it's being caused by the immune system going a little bit haywire and attacking cells in the body that it shouldn't be targeting, which is really fascinating.
Kat - It's very interesting because this affects about 1 in 3,000 people and the cause of it has been a complete mystery. It just causes people to randomly fall asleep, they have a lot of muscle weakness, a lot of problems. So, what's the connection then with flu and the immune system? What's going on here?
Nell - Well, it seems that what the immune system is doing in people with narcolepsy is it's actually latching on to a molecule that's found in some types of brain cells, some types of neuron. The reason it's doing that is because it's found a similarity between this particular molecule and another one that's found in a type of flu called H1N1. So, it's an accidental kind a bit of mimicry, almost, that's going on.
Kat - So, the immune system thinks, "That's a bit of flu" and starts destroying whatever it is. But in fact, it's these brain cells that are involved in wakefulness.
Nell - Exactly. So, the immune system is going wrong. It's trying to do its job. It's trying to track down harmful pathogens in the body and destroy them which it works very well at doing that. But clearly, in this particular instance, it's being a little bit too clever. It's very specific. It's found this bit of a protein that just randomly happens to match this part of the flu protein. It's also in these brain cells and that means that immune system is actually attacking the brain cells and completely getting rid of them.
Kat - This sounds like very interesting coincidence, but is it actually relevant to people? Do we know that?
Nell - Well, it looks like it could be interesting because it could mean that researchers can find a better way to help tackle the disease. They may even be able to find a way to test for narcolepsy using a blood test, which would be really valuable because as you were saying, we haven't known up until now what the cause is. So, it's very, very difficult to figure out who might have it, why they've got it, all these different types of things that we need to find out if we're going to be able to help people a bit better.
Kat - I thought it was really interesting that they highlighted that in 2010, there was a study in China that showed there was an increase in narcolepsy in a certain group of children living in areas where there was this big H1N1 flu pandemic. It's certainly very interesting link that needs more research
Nell - Yeah, it's really interesting. There's also some evidence from Scandinavia that when an H1N1 vaccine was used there, it actually led to a small increase in narcolepsy. It seems to be because they were training the immune system to react to H1N1 and it was also causing it to react to these cells in the brain at the same time. So, interesting to see how that can work and how the immune system can have these different effects across the body.
Kat - And certainly, informs the development of flu vaccines as well. It would be very interesting to see if these kind of accidental immune responses are behind other brain disorders. Things like schizophrenia which has been shown to have a link with autoimmunity.
Nell - Yeah, absolutely. I mean, it just seems like more and more, the immune system has a part to play in many different kinds of illness, many types of reactions to different diseases. I always think this type of stuff is really fascinating.

12:05 - Flu response variations
Flu response variations
with Nell Barrie
Kat - There was another flu story I saw from scientists at the University of Melbourne and they've published this in PNAS this month. They've discovered a genetic marker that can predict whether patients will have a more severe response to flu. This is a new strain of flu - H7N9 - that's currently found in China. Tell me a bit more about this research.
Nell - So, this seems to be to do with the way a particular person will react to a type of virus. If they've got higher than normal levels of cytokines as a result of a type of genetic variation then they can get a really severe level of infection from some types of flu. We all know that flu affects people in different ways. It's always a nasty illness, but some people get really seriously ill. We know that many people can die from some strains of flu. So, being able to say which people will react severely is absolutely crucial if we're going to find a better way of tackling it and making sure that future pandemics aren't such a big threat.
Kat - It's interesting that what the researchers found is people with this particular genetic variant, it's a protein called IFITM3 for the specialists out there, leads to what's called a cytokine storm. This is where your immune system just goes crazy. It's creating all these molecules that stir up the immune system. So, finding ways to understand that, I think will be very important in managing these kind of responses.
Nell - Absolutely. I mean, cytokine storms can be causing different types of situations as well. So, anything that teaches us more about that could be really beneficial to stopping people and getting affected by that type of thing.

13:30 - Mice on drugs
Mice on drugs
with Nell Barrie
Kat - And the final story I thought was just quite amusing. It's research published in the journal Science from a team of US researchers including some at the Jackson Lab which is a huge mouse facility. They've basically been putting mice on drugs.
Nell - Yes, indeed. Actually, what they found is very interesting. They were looking at the way that mice respond to things like cocaine and methamphetamine using a strain of mice that's been bred for a long, long time. But actually, what's happened over almost 100 years of this particular mouse strain being used is that different subpopulations have emerged. So, although one lab mouse may look very much like another, they are in fact different in some quite crucial ways. By looking at the way they responded to these different types of drugs, they've discovered that there are quite subtle but important changes within the same types of mice.
Kat - I think it's a bit sad they used black mice rather than "Walter White" mice - a bit of a Breaking Bad reference... But this research really does highlight the importance of knowing what kind of mice you're dealing with. A lab mouse is not a lab mouse the world over. The mice that they were looking at came from the founder of the Jackson Laboratory, Clarence Cook Little and this was back in 1921, and these mice are used all over the world. I remember seeing some research done in the '60s that said, "Researchers, you need to be aware that there's a lot of differences in how mice strains that seem to be very similar respond to things like drugs". So, it is a message for researchers using animals that they need to be very careful about what strains they use.
Nell - Yeah, absolutely. The scientists highlight that, especially when you're looking at things like behavioural data because it's so hard to unpick exactly what's going on there. So, knowing about these very, very small genetic differences between the different mice that might be being used is really crucial. Actually, what they do at the Jackson Laboratory is they try to make sure that they're kind of going back and refreshing the population of mice every so often so that they know exactly which strain they're using, they know exactly what types of changes might be there.
Kat - Yeah, it's interesting. They use frozen IVF embryos to do that. So, they're always going back to this stock of frozen embryos and they know exactly what they're like. But certainly fascinating research, and probably one to watch in the future.
15:37 - Gene risk for diabetes found
Gene risk for diabetes found
Writing in the journal Nature, an international team of researchers in Mexico and the United States has uncovered a new gene variation implicated in a raised risk of type 2 diabetes, particularly among Mexican and other Latin American people. Carrying a single high-risk version of the gene, called SLC16A11, increases the chances of developing type 2 diabetes by around 25%, while inheriting two copies of the risky version - one from each parent - gives a 50% increase in risk.
The higher risk version of the gene has been found in up to half of people with Native American ancestry, including Latin Americans, potentially accounting for a significant proportion of the known increased risk of diabetes in these people. It's in about a fifth of east Asians, and is relatively rare in people from Europe and Africa. the gene was previously missed in other genetic studies of diabetes which focused mainly on European or Asian people, highlighting the importance of doing this kind of research across a wide range of populations.
The gene seems to be involved in controlling the levels of a type of fat that's involved in diabetes, and the researchers hope their discovery will lead to improvements in the ways in which the disease is detected, prevented and treated.
16:52 - Genes, stress and death
Genes, stress and death
Researchers in the US have discovered that a gene variation that makes some people extra-sensitive to stress could also boost the risk of heart attack and death in people with heart disease. Publishing their findings in the journal PLOS ONE, the team focused on variations in the 5HTR2C gene, which encodes a serotonin receptor that's involved in responses to stress. People with a particular variation of the receptor are highly susceptible to the effects of the so-called 'stress hormone' cortisol.
Cortisol is also involved in inflammation, metabolism and other factors that play a role in heart disease, so the scientists analysed DNA from more than 6,000 patients with heart disease. they found that people with the gene variation that increased their stress responses also had the highest rates of heart attacks and deaths over the six years of the study, even when other factors like age, weight and smoking were taken into account.
The scientists think their research could help identify people at greater risk of heart attacks, who might benefit from more intensive prevention and treatment options.
17:52 - Jaw defect genetic model
Jaw defect genetic model
Our faces are sculpted in the womb, by cells called neural crest cells coming together and changing into bone, cartilage, nerves and more. Problems with this process lead to facial deformities, which can be extremely serious if they prevent a baby from feeding properly. Writing in the journal PLOS Genetics, a team of US researchers has now created a mouse genetic model that mimics a human condition called syngnathia, where babies are born with fused upper and lower jaws, and other problems with their faces.
The team focused on a gene called Foxc1, which is switched on in neural crest cells in a developing embryo. Using genetic engineering techniques they created mice lacking the Foxc1 gene, and found that the mouse pups had facial deformities that were very similar to those seen in babies with syngnathia.
Although syngnathia is relatively rare, around a third of all birth defects involve the head and face, so the researchers hope their new model will help to shed light on the origins of more common forms of facial deformity too.
19:05 - Prof Maria Bitner - childhood deafness
Prof Maria Bitner - childhood deafness
with Prof Maria Bitner-Glindzicz
Kat - You're listening to the Naked Genetics podcast with me, Dr Kat Arney. Now it's time to delve into some of the issues around the genetics of childhood deafness with Professor Maria Bitner-Glindzicz (glinjich) from UCL's Insitute of Child Health. I started by asking her where we are at the moment with hunting down genetic causes of hearing loss in childhood.
Maria - We can only look for really the most common genetic forms of hearing loss in childhood. So, these are one or two, maybe three or four genes that are commonly mutated in childhood onset hearing loss or genes which give rise to a distinctive clinical picture which we can pick out and identify clinically as belonging to a subset of genetic causes.
Kat - Now obviously, it's only useful to identify something if you can actually then do something about it. It's not very useful to say to a child, "You've got this gene problem, sorry." What can we now do with that knowledge, knowing that a child has a particular gene fault?
Maria - In terms of treatment, treatment is still very limited, but the kinds of benefits that might come out of having a molecular cause are that you might know that one particular type of deafness is associated without the medical problems. In which case, that knowledge will be useful because having made the diagnosis, you would then go and look for the other medical problems and put in place maybe treatments or screening for that. I'm thinking of syndromes so, syndromic causes of deafness. Actually, treating the deafness itself is more difficult, but if you knew that a particular type of deafness was likely to progress or get worse, then again, you might be more likely to refer the child on for something like a cochlear implant assessment. So, in that respect, there are some benefits to be gained. The other benefits really are in terms of genetic counselling, so it might not be very interesting for the child themselves to know their cause of deafness. But for the parents, it might very important. If they're thinking of having more children then knowledge that their child's deafness is genetic or that it is or is not associated with other medical problem might have a major influence on whether or not they decide to have more children.
Kat - What sort of syndromes and types of hearing loss are we talking about with these genes that we do know about?
Maria - Well, some of them involve major clinical problems and these are just minor clinical features that just help you to make a diagnosis. So, one of perhaps the major syndromes would be something like Usher Syndrome where congenital hearing loss is associated with later onset, by which I mean teenage onset, for retinal degeneration that ultimately leads to blindness. So, that's clearly a very disabling condition and an important diagnosis to make for the reasons that I've just outlined. Other syndromic forms of deafness, maybe we don't have major clinical complications - so, something like Waardenburg syndrome includes the association of deafness but with unusual pigmentation of the hair or skin or eyes. That doesn't cause the person any medical problems, but it helps us to identify the cause, how it's inherited, and maybe to some extent, what the future will hold for that person.
Kat - Where do you see us going in maybe 5 to 10 years with moving from the knowledge gained in the lab, from the animal research and the big genetic studies that are tracking down these genes? Where would you like to see that heading in terms of how we can improve the outcomes for patients?
Maria - I think there will be much better diagnosis. So, the proportion of children and families in whom you can actually say, "Well, this is the cause", I think that's going to take another leap forward. I think, what we know about the time course of some forms of deafness I think is really also going to improve, because at the moment, although we know a number of genes that can cause hearing loss in humans, our clinical knowledge based on a handful of cases. So, once that handful then becomes hundreds of cases you get a much better idea of what that type of deafness involve, or will looks like, and what will happen over time. I think we will be able to - as a result of that - maybe predict better which children will get more benefit from cochlear implants. So, some children appear to do very well after cochlear implants and some do far less well. There might be lots of factors for that, but one of those factors might be the cause of their hearing loss. If you could subdivide what's at the moment a very mixed group of children into different groups and then follow them up, I think that will be very useful. And of course, I suppose ultimately, one aim is to prevent progressive deafness and maybe to treat some forms of deafness. I think once we understand better about why people are deaf then we can begin to tackle those much more difficult aims.
Kat - Slightly changing tack, one of the other interests of your research group is studying how hearing can be damaged through things like drugs and the genetic link to that. Can you explain a bit more about what's going on there?
Maria - Yes. We are interested in a particular type of hearing loss which, essentially, is a genetic predisposition to hearing loss after a group of antibiotics called aminoglycosides. So, if you have this predisposition and you have these antibiotics, you can experience a really rapid deterioration in your hearing which is irreversible. Of course, that begs the question of, "If you know who these people are, then surely, you just don't give them these antibiotics." Of course, that's the obvious thing, but one has to try and work out how they cause this type of hearing loss and the best way of trying to pick up people who are sensitive.
Kat - It does seem that more and more, we are understanding how our genetics make us almost unique in how we respond to the drugs and treatments that are available. Do you think this is going to be an increasingly important field in the future?
Maria - I think it has to be. I think all of those drug reactions that one learns about as a medical student and junior doctor, you're beginning to realise now that these are probably all genetically based. They are likely all to be explained by the genetic makeup of a person within the next decade. To me, that's really fascinating - all of these rare idiosyncratic drug reactions are likely to be genetic.
Kat - What first got you interested in studying deafness and hearing loss?
Maria - That's a very good question and, as many people do, I think you come to it in a rather roundabout way. I was very fortunate when I came here to the Institute Of Child Health as a young geneticist that an interest in genetic hearing loss had already been established by some of the researchers who preceded me. So, it was very easy to see that it was going to be a field that was opening up.
We had an excellent team of paediatric audiological physicians who'd built the department here really from nothing. We were one of the first centres to do paediatric cochlear implantation. We had a fantastic clinical genetics department, and it was realised that actually, genetic hearing impairment was an area that had been really neglected in terms of clinical studies. So, it was an ideal field to enter really. Of course, as soon as you enter into a clinical or a research field, you meet other people who inspire you, you meet patients that you remember or whose stories somehow touch you really and spur you on to try and find out more.
Kat - That was Professor Maria Bitner-Glindzizc from UCL.

28:00 - Gene of the month - Headbobber
Gene of the month - Headbobber
with Kat Arney
And now, it's time for our Gene of the Month, and this time it's Headbobber - a genetic fault explored this year by Professor Karen Steel and her team, who we heard from earlier. Headbobber mice have characteristic ear problems including bobbing their heads (obviously), going round in circles, balance problems and deafness. Although the precise gene fault hasn't been tracked down yet, it's due to a missing portion of mouse chromosome 7, which contains three genes, as well as some control switches for nearby genes.
These genes seem to be important for the growth and organisation of the inner ear in the womb, which creates the parts responsible for hearing and balance. Importantly, this mirrors a genetic fault in humans with similar hearing and balance problems, which are due to a missing portion of chromosome 10 - the comparable region to mouse chromosome 7. The researchers hope the Headbobber mice could be a good model for deafness caused by these gene faults, and help to shed light on the condition in humans.










Comments
Add a comment