Q&A: Moon Landings and Making Medicine
In this week’s programme, it’s Q&A time! Coming up, we’ll find out: what can we learn from invisible measurements in space, how scientists discover potential new medicines made by plants and why green energy might be more costly to the consumer... Yep, we’re answering science questions you’ve been sending in! We've assembled the very best experts to help get to the bottom of it all: climate researcher Ella Gilbert, climate economist Gernot Wagner, enthnobotanist Cassandra Quave, and public astronomer Matthew Bothwell...
In this episode

- Would Captain Scott still recognise Antarctica?
Would Captain Scott still recognise Antarctica?
Ella Gilbert leads us on a polar exploration...
Ella - In as much as one can recognise a vast continent that looks very similar, maybe. Chunks of ice shelves, for example, have broken away, we get lots of year-to-year variation in the amount of sea ice that covers the ocean around Antarctica. In 1995 and 2002 we lost two entire ice shelves on the Antarctic Peninsula, which is a bit that sticks out, and lots of ice shelves regularly have gigantic chunks breaking away and turning into huge icebergs that circle the continent. But in terms of the stuff that's on land, I would say it probably looks relatively similar.
Chris - A vast expanse of white?
Ella - Yes.

05:23 - Can we use atmospheric carbon for food?
Can we use atmospheric carbon for food?
Climate scientist Ella Gilbert and geoengineer Gernot Wagner weigh in...
Chris - This week you've probably been watching the headlines here in the UK, food production is apparently under pressure due to a lack of CO2. Listen to this...
NEWS READER - The threat comes after two huge fertilisation plants in Teesside shut down due to gas price rises. CO2 is a byproduct of fertilisation and the supply constraints could be felt across the food and drink industry.
Chris - On that subject, Cornelia is wondering why the shortage of CO2 should be causing problems in the food supply chain? At the same time, we're continuously being told there's too much CO2 in the atmosphere. We've got too much of the stuff. What's going on?
Ella - I know it's a bit of a head-scratcher isn't it, that you can simultaneously have too much CO2 and too little. First off, CO2 emissions or carbon dioxide emissions are the primary cause of climate change. CO2 is a greenhouse gas. It forms in the atmosphere which makes it a problem, which is why we're constantly being told that we need to reduce our CO2 emissions so that we can have less of an impact on the environment. When we're thinking about the food industry, it has a variety of different uses. That's for things like carbonated drinks and, as I only learned this week, for killing pigs. These sorts of uses require a lot of CO2 and you have to manufacture it. Lots of those plants manufacture it as a by-product of other processes, things like fertiliser. In the atmosphere CO2 is in relatively low concentration. At the moment we've got 420 parts of CO2 for every million parts of air. To actually concentrate that CO2 would actually be very challenging because we don't have the sorts of geoengineering techniques that I'm sure Gernot will be talking about later, like carbon capture, to actually distill them into a really concentrated form.
Chris - One big source is methane, natural gas, isn't it? We rip away the hydrogen off of it and turn the carbon that's in it into carbon dioxide. Gernot, that seems something of a contradiction. When we're thinking we're trying to get rid of CO2, why can't we use the stuff we've got in the atmosphere? We're told that we've increased the amount of CO2 in the atmosphere by enormous amounts since the industrial revolution. If there's so much there, why is it so difficult to just grab it back?
Gernot - As we speak the very first demonstration plant like this is operating. It's a Swiss startup, a Swiss company, operating in Iceland, taking CO2 out of thin air and concentrating it. It costs them about a thousand euros per ton of CO2, which is incredibly costly if you look at it from lots of different perspectives, and frankly it doesn't pay to do that for food, it is too costly. But costs are only going to come down - you need to start at a thousand to get a hundred eventually, and so on. The technology does exist, it's just very pricey.
Chris - How does it work?
Gernot - In some sense, in a chemical sense, it's literally reversing the process that we use when we burn fossil fuels and release the CO2. That alone sounds energetically very costly. There is a reason why this Swiss startup is operating in Iceland. Why? Because there's a lot of cheap geothermal energy, cheap and low carbon, zero carbon. Those are the key ingredients here in this process.
Chris - Is that the way it's going to go then? Do you think that what we'll end up doing is we'll put CO2 scavenging plants where we're currently seeing a waste of energy things, like hot rocks and volcanoes, there'll be certain countries where their growth industry in the future could actually be pulling CO2 out of the atmosphere and then turning it into stuff?
Gernot - Here's hoping. Way too many people who make too much money taking fossil fuels out of the ground are burning them, and of course, to be clear, this process itself is useful, it provides energy. But by now we have better ways of doing that, cheaper ways of doing that; solar photovoltaic, solar PV, is the cheapest form of electricity in history. There are better ways of doing that and we should stop burning fossil fuels and there is already too much CO2 in the atmosphere. We should, and frankly will be, taking that CO2 out at some point, at a cost.

10:04 - How might climate change affect herbal remedies?
How might climate change affect herbal remedies?
Ethnobotanist Cassandra Quave explains how medicinal plants could be at risk...
Chris - Cassandra, you go all over the place hunting for plants that have exciting medical properties. Presumably one place you can start is to ask the locals?
Cassandra - Yeah, absolutely. That's one of the main ways that ethnobotanists learn about the uses of plants is through interviewing locals or also looking at historic literature. We've looked at records going back to the 1600 of plant uses in different parts of the world
Chris - One thing that's got me concerned is that you're doing this work, but the plants that those locals know about may, thanks to the effects of climate change, not be there for much longer
Cassandra - Absolutely. We're facing a crisis on a global scale when it comes to the impact of climate change on the availability of many of these plants. I can give you some statistics on this. We know that there are around 374,000 species of plants on earth and roughly 9% of those are actually used as medicines by people. When we think about plant based medicines in the west, we often think of herbal teas or dietary supplements or these kinds of commercialised versions of these. Actually billions of people across the globe rely on plants as their primary form of medicine. They're acquiring them through harvesting them in the wild during peak seasons of when they're in flower or fruit and then storing them throughout the year in their home, or growing them in their gardens. For those wild harvested plants there are challenges that are rising as we see ecosystems impacted. When you think about the optimal climatic zone, the optimal elevation point at which different plants grow, as climate change and environmental factors encroach you start to run out of mountain as the plants migrate up. Yeah, there are some serious problems with supply.
Chris - Somewhere I read that about a third of the drugs that are in the top 10 of things that are in your average doctor's bag or your average hospital formulary have their direct roots in nature and in plants
Cassandra - Absolutely. The World Health Organisation has a list that they come out with. It's called the World Health List of Essential Medicines. If you go through that list you can see many examples of drugs that we use today to treat cancer, heart disease, pain, malaria, infection, all types of different diseases and the chemicals that are used in those drugs and the structural blueprints for many of those cases were originally derived from plants.
Chris - Why do plants need an anti-malarial though? Just to take an obtuse example, what's it doing in the plant? Is it just luck that the same molecule, when you put it into us helps to treat malaria and in plants it does something different? Or are there some shared characteristics, so plants make these things to ward off the same sorts of problems that we get?
Cassandra - First we have to set this idea that plants are really some of the best chemists that exist on earth. They have this amazing slew of compounds they produce. Even in a single leaf tissue you may have hundreds of distinct molecules. Some of those molecules are useful for processes of photosynthesis and just simple growth and reproduction. But many of them are what we call secondary metabolites. These are the compounds that plants use to defend themselves from pests and from herbivores that get a bit too greedy as they munch on their leaves. Any kind of animal that gets a bit too munchy, plants can actually change their metabolism. They can actually upregulate and start to produce higher levels of poisonous compounds in their leaves to deter or repel other organisms that are harming them. In the same sense they can release compounds that attract pollinators and seed dispersers. I like to use the example of the corpse plant. I don't know if the audience is familiar with that, but this is sometimes in botanical gardens and they call it Mr. Stinky, it's a titanarum. When it blooms, it has this amazing smell of a rotting corpse.
Chris - I like Ella's face when you said that! Said it all, but they did have one of those at Cambridge University. They don't flower very often do they? It took decades before this one was big enough to flower, apparently the smell was really something to write home about.
Cassandra - Exactly. They're found in the wild in Indonesia. I came across one, not in flowering state but in its leaf form once in the forest. It was just amazing to see it. I felt like I was a fan girl. It poses this question of why does a rose smell like a lovely rose? Why does this titanarum smell like a rotting dead body? It all comes down to those chemical signals. They have different pollinators. Different organisms need to come to them because plants are sessile, they can't get up and move away from threats or go towards resources that they need. They rely on these chemical signals to really recruit or repel other players in the ecosystem.
Chris - Amazing stuff, Cassandra, thanks very much.

16:42 - Astronaut or rover?
Astronaut or rover?
Univeristy of Cambridge public astronomer, Matthew Bothwell stars in this one and explains why the moon will likely become a more common destination for our astronauts over the next century...
Chris - In the last few days NASA have announced the landing site for another rover. This one's going to be called Viper interestingly, and it's heading to one of the coldest places in our solar system, which is, Matt?
Matthew - The dark side of the moon
Chris - There you go. Not just a pink Floyd album. It is the south pole of the moon. That's where they're going. It's planned for 2023. Not NASA's only foray to our nearest neighbor out in space though, because there's the intention to land another crew member on the surface of the moon in 2024. This has got Joel thinking. He says, 'There's been a lot of discussion concerning a return to the moon. So why are we going back? Why can't we just send a Rover for that? Why send a human?'
Matthew - It's a really good question. I think there's a short term answer and a long term answer. In the short term it's useful to send people rather than robots to the moon because there's only so much you can do remotely. Human beings are really good at being adaptable and flexible and responding to things in the moment. If you notice something interesting looking on the horizon, or maybe you have some lunar dust on your experiments you want to clear, sorting that stuff out is trivial for humans. You can just wipe the dust off your experiments. If a robot hasn't been specifically designed to solve these problems it's going to run into some real difficulty. There are fiddly jobs that robots just can't do very well. For example, even to this day we don't really understand how heat from the interior of the moon diffuses out to the surface. That's because the astronauts in the Apollo mission tried to drill holes and their technology wasn't very good. They had trouble drilling holes. It's just too fiddly a job for a robot. We're going to have to send a person there to do it that way.
Chris - Why are we interested in heading back to the moon at all? Haven't we got bigger planetary fish to fry these days like Mars?
Matthew - We absolutely do but I think the first step towards frying those planetary fish is going to the moon in the coming century. We want to be exploring the whole solar system. We're going to Mars and that's just the beginning, right? We want to be going beyond Mars and exploring all of our cosmic home. I think human missions to the moon are a great proving ground for all the technology that we're going to need to help us along the way. It's very hard to test equipment in the environments on Mars, but the moon is basically next door in terms of space. It's a really nice first step to our coming adventures.
Chris - Is it a jumping off point? because some people have speculated that we put a base on the moon and then it's much easier to go places from there, rather than having to keep getting off of earth into space and then on to our next destination?
Matthew - True, Earth is a big planet, right? We're sitting at the bottom of a very deep gravity well and that's why you need such an enormous rocket to blast off into space. Gravity on the moon surface is about a sixth of the gravity on the earth surface. Getting into space from the moon is much easier. We're building these permanent installations, like a moon base, and that's going to be an orbiting space station around the moon a bit like the ISS, and getting into space from there rather than from earth should be much easier in the future.
Chris - Do you know where they're going to build these bases? Will we be able to see them from Earth? Presumably they will want them on the side facing us so we can talk to people easily because if they were around the other side they wouldn't better talk to us so easily!
Matthew - Yeah, that's exactly true. Any base with people in it is going to be on the near side of the moon, so it can radio Earth. There are reasons to go around to the far side of the moon as well. There are plans to build a radio telescope on the far side of the moon, which is going to be one of the quietest places in the solar system because you can have thousands of miles of rock between your radio telescope and the noisy radio Earth. In terms of exploring the distant universe we might be going to the far side. I think we're going to be exploring just more of the moon in the future in general.
Chris - Out with one of your big telescopes, doing your public astronomer bit, would you be able to see the settlement or would it be just too small?
Matthew - I think the settlement stuff they've planned will be too small to start with, but you never know what the future may hold. If there ends up being a permanent settlement of people on the moon, then in fifty years, a hundred years, it really might start getting quite noticeable.
Chris - Watch out, you might have Matt staring at you future moon inhabitants.
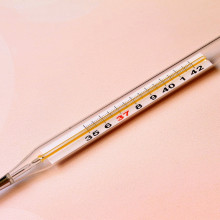
20:55 - Should we trust old climate measurements?
Should we trust old climate measurements?
Ella Gilbert from the University of Reading gives us the lowdown on old climate data...
Ella - That's a really good question. Generally, with climate modelling, we try to integrate as much real world data as we possibly can to get the best kind of picture of what conditions are like when you press go on the model. And people have been collecting measurements of temperatures for a really long time. So it's one of first things that we can measure. But technology has improved quite a lot thankfully since the 1800s and 1900s, when measurements were taken using relatively cruder techniques. For example, using glass mercury thermometers. One of my favourite stats is that before the 1940s to measure sea surface temperature people would throw a bucket overboard on ships, drag up a bucket of water and stick a thermometer in it. So you can imagine that in the process of getting from the sea up over the side of the ship, it would maybe change temperature a little bit.
So not exactly the most accurate, whereas now we have different techniques: we can use underwater buoys and robots to collect our data; we have automatic weather stations these days, which can record temperatures etc. So it's gotten a lot less old school and the ways that we collect those temperature measurements has changed. So stations where we record these temperatures: they might have moved, they might change the hour of the day when they collect the observations, and all of these things actually have an impact on the values that are recorded. You can essentially get a step change when something changes in the way that the measurement is taken. So you have to adjust for all of these things, and there's some algorithms devised by cleverer statisticians than I that account for this. I think they call it a process of homogenisation, where this accounts for the sort of step changes we see when a station moves or it changes the hour that it collects information at.
There are a few massive data centres that collate these long time series that get fed into climate models. One of them would be Berkeley Earth, there's one at the University of East Anglia's Climatic Research Unit, NASA also has one of these. And all of the big kind of centres that collect these temperature records have different methods of doing it, but it's essentially the same thing. If we're thinking about something like a change in the way we collect data, the principle is that you can assume that climatic changes are broadly similar on a regional scale. So that if you're seeing some really dramatic jump in one station, you can assume that that's to do with the instrumentation changing rather than a very sudden change in the climate.
Chris - So Chris's point that our predecessors - scientists of yesteryear - may well have recorded things a bit less accurately than we would today, and possibly less precisely as well. There may well be a bit of variation, but the trend is your friend, because if they did it consistently and they were a bit off consistently, it's the signal changing that matters, not so much the absolute number?
Ella - Exactly. And there's always going to be more uncertainty in yesteryear, because first of all, there were fewer measurements being made and we know that the techniques were slightly less accurate.

25:17 - Could fixing the climate impact stargazing?
Could fixing the climate impact stargazing?
Gernot Wagner of New York University explains how volcanoes could provide inspiration for a climate-change solution, and considers with the help of the University of Cambridge's Matthew Bothwell whether this could lead to unintended consequences...
Gernot - Maybe - we should probably take this outside.
Chris - No, don't do that. We need to record this programme.
Gernot - But just to be clear, that might be one of the smallest possible impacts or risks of solar geoengineering. I mean, it might change how the sky looks ever so imperceptibly.
Chris - Did you want to tell us actually what you would do then? Say you wanted to cool the planet, to do it quick and do it safely, what would you put and where?
Gernot - Here's what volcanoes do: in 1991 Mount Pinatubo erupts in the Philippines and in 1992 global average temperatures are about a half a degree centigrade cooler than they would have been without that volcanic eruption. If you throw tiny reflective particles into the stratosphere, into the lower stratosphere, somewhere around the equator, those reflective particles within weeks spread around the Earth and for a few months, for a year, maybe a year and a half, reflect sunlight back into space.
Chris - But what are those particles? Because obviously volcanoes are chucking out sulphur. For example, you could then get acid rain, couldn't you? Then obviously you're robbing Peter to pay Paul, because you'll have a cooler planet, but then you'll have dissolved everything on the ground. How are we going to do this safely then?
Gernot - It is sulphate aerosols - often that's the most prominent example. Now you don't get acid rain for the simple reason that up in the stratosphere there are no clouds. So as many problems as they are with solar geoengineering, acidifying the oceans of the world even further than we already do... Just to be clear, we do a lot of that by adding CO2 to the atmosphere, and that's a real problem. And, no, solar geoengineering does not address that root cause. But yes, there are lots and lots of unknowns, lots and lots of uncertainties. And frankly, the name of the game here is that we should be doing the research, right? Nobody sensible is calling for actually deploying any of this. And now we shouldn't, what we should be doing is figuring out whether it could work where the benefits as far as they are - and there are great benefits here, e.g. lowering global average temperatures - whether they do outweigh the costs, the risks, the uncertainties.
Chris - Have astronomers not got quite crafty ways of subtracting wonkiness in the atmosphere? So when light comes through the atmosphere, it causes stars to twinkle - the stars look like they're twinkling because light is bending anyway as it comes through the atmosphere. Have you not got clever adaptive ways of getting around this?
Matthew - Yeah, we absolutely do. Astronomers use the slightly stupid word 'seeing' to refer to this kind of twinkly bendy thing that light does when it comes through our atmosphere. And yes, we do have clever ways of dealing with it. Basically what we'd have to do is just make a model of the atmosphere - we live under a hundred miles of turbulent, wet gas - and if we can understand what the turbulence is doing, then we can subtract that out and see clearly. A lot of the issue we have with things going into space is not so much about the bendy twistyness of light as it goes through the atmosphere, but just particular wavelengths being blocked or even overwhelmed. The Starlink project, so Elon Musk's plan to put a bunch of satellites in space and broadcast internet around the world - that could be catastrophic for radio astronomy. Because with those things it's like putting a bunch of mobile phones or internet routers in orbit. Any radio telescopes on Earth might just be absolutely bombarded by these signals from outer space. If it's for a good cause, it might be hard to say no. The night sky is very wonderful and obviously astronomy is important, but if it's a choice between that and fighting climate change, I think I know what side I'd be on.

32:18 - Autumn quiz: a time for change
Autumn quiz: a time for change
Ella Gilbert, University of Reading; Gernot Wagner, NYU; Cassandra Quave, Emory University; Matt Bothwell, University of Cambridge
Our teams this month are atmospheric climate scientists Ella Gilbert and astronomer Matt Bothwell, and climate economist Gernot Wagner and ethonobotanist Cassandra Quave.
Chris - Now as we always do on these programmes, we like to pause halfway and test the mettle of our panel with a quiz. And of course you at home can try it and see if you can have a bigger brain than they do, because you are competing, everybody, for a prize beyond prize this week. It is the Naked Scientist 'Big Brain of the Week' award that you're going to win. So there are going to be two teams and team one is Ella and Matthew, team two, Cassandra and Gernot. Now there are three rounds to this quiz and conferring is actively encouraged because each team's going to get different questions. So do talk between yourselves and establish what you think the answer is. We won't be awarding marks for - unlike your maths paper at the exam - showing your working. But we do like to hear it all the same. For you at home, this is your chance to see if you can outwit our panelists.
Chris - Now because the seasons are changing and here in the Northern Hemisphere, we are heading into autumn, we thought a good theme for the quiz would be 'change'. So let's do round one first: 'too hot to handle'. This is a change in temperature. Question one, which of these elements will melt in your hand? A) gallium B) bromine or C) mercury?
Matt - I think bromine is a gas at room temperature. So it's not going to be that.
Ella - I wouldn't fancy handling any mercury.
Matt - Dredging this up from the depths of my memory, it might be gallium. I'm not sure I'm more than 50% sure.
Ella - Well, that's more sure than I am.
Chris - Is that what you're going for?
Ella - Yeah. Why not?!
Chris - Yep. Well done. It is gallium. Gallium is a soft metal. It's a silvery color as most metals are, and it will melt to a liquid at any temperature which is higher than 29.76°C. Of course, your body temperature at 37°C, give or take, means your hand temperature is capable of melting gallium. Bromine and mercury are already liquids at room temperature, so if you have them in your hand, unless they're starting off extremely cold and that wouldn't be pleasant to hold, they wouldn't be naturally melting in your hand. Right! One so far to Matt and Ella.
Chris - Team two: Gernot and Cassandra, here's your question. Verkhoyansk in Russia is the place on Earth with the greatest range between its highest and lowest recorded temperatures. Is it A) 66°C, B) 86°C or C) 106°C? What do you think?
Gernot - Okay. So a hundred is a bit high, right? We are talking minus 40 to plus 60. That doesn't quite sound right. So how about the middle one here?
Cassandra - Yeah, I concur. We'll go with that. We'll go with the middle.
Chris - You're going 86 degrees. I'm afraid, no, the answer is actually 106. Verkhoyansk is home to over a thousand people. It holds the record for being both the hottest and coldest place at different times. And it has the widest temperature range, therefore. It's recorded a scorching 38°C on some occasions and a brisk, shivery -68°C on others. So 166°C.
Ella - I was sitting on my hands the whole way there because I knew that.
Chris - Oh, there you go. Now you know why we didn't make you team two!
Gernot - That seems hot!
Cassandra - I'm thinking of their closet? What kind of clothes? I mean, how do you prepare for a year like that?
Ella - Two wardrobes!
Chris - You need two wardrobes, quite right Ella. Right. Ella and Matt back to you. Your question: salmon are famous for swimming upstream from the ocean along rivers in the autumn to reach their spawning beds. This is a race called the salmon run, but what's the average speed that sockeye salmon doing that migration do on the Fraser River? Is it 0.9km/hr? Is it 2.4km/hr? Or a speedy 6.8 km/hr? What do you think?
Matt - This feels like a Monty Python question to me.
Ella - It really does! It depends how fast the river is! I feel like maybe the middle one.
Matt - Yeah. Like based on nothing but intuition, just throw the data at the map, the middle one...
Ella - 6km/hr is fast.
Matt - Like that is pretty fast rate.
Chris - Well a fast shark or a tuna will do 60 or 70 km/hr.
Ella - Yeah, but this is an average, right? Are they going like uphill up rapids? That's what you always see in the nature docs, isn't it? Bears leaping at them.
Matt - Yeah, faster than walking pace seems fast for an average.
Chris - Matt's going for a more sedate two and a half kilometres an hour. Is that your answer?
Matt - I'm imagining a pretty chilled out salmon going down the river, you know?
Chris - The public astronomer says 2.5km/hr seems like a sedate speed for salmon seems good. And you're absolutely right. Yeah. It is actually 66.75 centimeters per second. It's been demonstrated that the most efficient speed to travel at, if you are a sockeye salmon, is 1.8km/hr, but that wouldn't work if you were going to miss the festivities and not get to your spawning beds in time. So that's why they swim a slightly less sedate two and a half kilometers per hour.
Chris - Cassandra and Gernot, back to you. Your question is: Mars has a longer orbit around the Sun than we do here on Earth. And Matt you'll know why you didn't get this question. It means that the seasons are longer on Mars. To the nearest whole number, how much longer are the seasons on Mars? Are they A) twice as long, B) three times as long or C) four times as long as they are on Earth?
Cassandra - I don't know. I wonder how far is Mars from Earth? Maybe that could help. I know it's really far!
Matt - Miles is tilted by about exactly almost the same tilt as Earth. So it has seasons for just the same reason.
Cassandra - That's great. Can we phone a friend? I want to have Matt!
Chris - Matt's not going to help you here I'm afraid!
Gernot - Wait, so one of the options was twice as long, right?
Chris - You've got twice as long, three times as long or four times as long. What do you think?
Gernot - Twice as long.
Chris - You're going twice. Do you agree, Cassandra, you're going twice?
Cassandra - We'll go with twice
Chris - Correct! One Martian is 687 days, and that's almost twice as long as an Earth year, so the seasons are roughly twice as long. As Matt says that Mars is tilted in the same way with roughly the same inclination that the Earth is, which is why it has seasons, and therefore they're roughly in proportion to the Earth seasons as well.
Gernot - So is that why Elon [Musk] wants to go there just to have every year be twice as long?
Chris - The thing is you come home and you're only aged by half as long. If you believe that you believe anything.
Chris - Right. Round three. This round is called 'a change in time: it goes by so slowly'. Question to Ella and Matt. Getting a handle on different time zones is important in the time of online communication, but over the last couple of years, no one has really made any drastic changes of them for the sake of zoom calls for instance. But that wasn't the case in 2011, when which island decided to jump ship and skip a day out of their calendar, which the victim was the 30th of December to jump across the international date line and become one of the first countries to see in the new day of that year and the subsequent new year, of course, rather than one of the last. Was it Samoa, Fiji or Tonga?
Ella - I remember this happening and I can't remember which one it is.
Matt - Yeah, same. I watched a YouTube about this quite recently because they wanted to be able to do business with Australia, right? Because I think the problem was it was Friday for the islands and it was Saturday in Australia. So they couldn't do business with them.
Ella - I can see why that was a problem. I don't think it's Fiji. I don't. I'm not basing that on anything sensible. That's just a gut feeling for Samoa, but...
Matt - Yeah. Then let's go with your gut feeling.
Chris - And it's a good call! It is Samoa! Well done. The dateline runs across the Pacific Ocean. It separates one day, literally, from another and American Samoa is on the eastern side of the date line and that means that those two regions are separated by a whole day, even though they're only 30 miles apart. Well, that puts you in a very much unassailable position, I'm pleased to say, for you Matt and Ella. It does mean that for Cassandra and Gernot, it is a winning streak, not for you this week, but I'll give you the last question anyway, because this is your opportunity to partially redeem yourself.
Chris - I hope you know about koalas. When a koala is born, it's about the size of a jelly bean. It's got no hair, no ears and it can't see. But how long is a koala's gestation period? In other words, how long is it pregnant for? 7-14 days, 28-35 days, or 63-70 days? What do you think?
Cassandra - Okay. Let's think about this. I think they're so tiny, right? And they kind of mature outside of...
Gernot - Yeah. Shorter, I believe, but frankly... My wife's a human gynaecologist, so I wouldn't be able to... What was the middle one?
Chris - Seven to 14 days, 28 to 35 days, or 63 to 70 days.
Cassandra - I mean, you go from a cell to like something that would be large enough. I would say like at a minimum would be 28 days, I think. Right. Like to get to a decent enough size to survive outside.
Gernot - With 28 to 35 for the prize?
Cassandra - Yeah. Yeah. I think so.
Chris - And it's a good call to make because it's the right answer. Yes, it is 28 to 35 days. Very well done. Unfortunately you are not the Naked Scientist Big Brain of the Week Award winners this week because that accolade goes to Ella and to Matt. So let's give them a round of applause. Well done guys. Excellent demonstration of knowledge of time and change.

41:51 - Can plants solve the antibiotics crisis?
Can plants solve the antibiotics crisis?
Ethnobotanist Cassandra Quave told Chris Smith about the role plants have to play...
Cassandra - I mean, we have a couple of problems that's really spurring antibiotic resistance. One of them is misuse of our existing antibiotics, not only with regards to overuse in the clinic but also massive use in agriculture. So we need to work on that. We need to work on also the economic models for developing antibiotics. But the main problem that we're focused on is really helping to fill that pipeline. We need new molecules, but we also need new approaches to dealing with infection. And this is where traditional medicine I think can really provide some important clues because even in our own work, we found that healers don't always treat an infection with plant products that kill bacteria. But a good example of this is our work on pepper tree and on chestnut. These are used to treat wounds, but they don't have growth inhibitory effects against the bacteria. Instead they shut down the ability of bacteria to cause harm. So I think from my perspective, yes, plants have a lot to offer in terms of novel chemistries. And they may actually open the doors to new ways of dealing with these infections.
Chris - That's very interesting. So rather than just kill them outright, you just basically render them a bit impotent so that the bacteria much less able to cause disease giving the body a helping hand?
Cassandra - Exactly. I like to liken it to, you know, taking the teeth out of the dog's bite, right? The dog is still there. He may gnaw on your arm, but I couldn't do much damage
Chris - That just licks you to death, which my dog seems to do. Fantastic.

44:48 - Arcane supernova in the sky
Arcane supernova in the sky
University of Cambridge's public astronomer, Matt Bothwell, does some sleuthing of astronomical proportions...
Matt - Fundamentally, what we have to do is just look at the patch of sky, which isn't always easy, right? Because these ancient peoples a thousand years ago, didn't always have very accurate ways of telling where things were in the sky. So we to kind of take their descriptions, look at the patch of sky and just try and piece things together by looking for whatever might've been left behind. The 1181 Guest Star has historically been kind of tricky. It was probably some kind of supernova. When something appears in the night sky and then lasts for a few weeks or something, and then fades away, some kind of supernova is normally a good guess. This one was a bit weird though, because it took months to fade away, which is much, much longer than normal. There was only one thing in that patch of sky that we used to know about that could have come from a supernova. It was a pulsar; the compressed core of a dead star called 3C85.
Matt - The problem is, when we aged it, it came to about 7,000 years old. So not a good candidate. It was a much, much older supernova. This new result, which got everyone really excited and has solved this 900 year old mystery, is that we found the smoking gun. We have found this new supernova remnant, and we combined it with a distance measurement taken from this satellite called Gaia. And we've worked out that it's about a thousand years old, which makes it perfect, right? It's a supernova remnant. It went off around a thousand years ago. And now we know these things, we can go even further and, and investigate it. So people at the time noticed that this thing was about the same brightness as Saturn, which is a pretty good yardstick. So now we know how bright it looked and how far it is away, we can work out how intrinsically powerful this thing was.
Matt - And it turns out it was actually pretty wimpy as supernovae go, which is kind of interesting. It was a wimpy little explosion, but it also took six months or something to fade away. That's a clue that it was something very, very rare. So our best guess now is that it was two white dwarfs crashing together and they've left behind something called a Wolf-Rayet star, which is a very, very hot star full of all kinds of interesting heavy elements. So yeah, it's going to need a lot more investigation because this was a very, very rare event, which has left behind something very exciting.
Chris - This is like stellar or cosmic archaeology almost isn't it?
Matt - It literally is, yeah. So all astronomy in some ways is the study of the past because the signals that we're seeing from the Universe have taken thousands or even millions of years to reach us. But this is taking that to another level because we literally are doing archeology. We're looking in the sky and then we're trying to put the pieces together and work out what's happened a thousand years ago.

47:42 - Is green energy more expensive?
Is green energy more expensive?
Gernot Wagner, climate economist from New York University, totals up the balance for users exploiting solar energy...
Chris - Michael wants to know why is green energy typically more expensive for the consumer? And is that true?
Gernot - Traditionally it has been, which is why we have this climate problem in the first place. Burning fossil fuels was cheap and available, largely because nobody paid for the pollution. Nobody paid for the fact that we are dumping 'gazillions' of tons of CO2 into the atmosphere. We should pay for that. Even without paying for it by now, solar PV is the cheapest form of electricity in history. Costs have come down so quickly. 40 years ago it was about 100 times more expensive than today. 10 years ago it was 10 times as expensive as it is today. In sunny locations, don't put your solar panel on the north side of the roof if you end the Northern hemisphere, assuming it's sunny, solar PV is cheaper than any other way of generating electricity and to be clear that's a big deal. That information comes from the international energy association, which is nobody's idea of an environmental group.
Chris - The install costs are very high aren't they, I was looking at this yesterday and the payback period for a domestic system might be 25 years in some cases, especially in a less sunny place like the UK.
Gernot - Of course, you are sitting in the UK, which I'm sad to say is not one of the sunniest places. That's okay. Sometimes there's an advantage of being in southern latitudes. Even here in New York, which isn't all that far south that payback period is a lot shorter. It depends on having a sunny location but the key thing here is yes, there's upfront costs, but then as soon as you have the panel you're basically printing money for free, you're printing electricity. There's no further costs other than wiping down the panel every once in a while. That's the big difference here.
Chris - But can I pick you up on that Gernot because a few years back in your wonderful country, there was this initiative to produce biofuels because everyone said, 'we need to make sure that we don't emit more new carbon into the atmosphere. We'll make fuel from the atmosphere, we'll grow plants.' The problem is that lots of good agricultural land was then used to grow plants with the result that then you had to ship in food from places with a higher carbon footprint than had you just grown the things at home. We also in danger of turning over quite a lot of agricultural land, especially in some countries which is at a premium, to turn it into a solar farm. On the one hand, you're incentivising people to produce cheap green electricity but then the carbon cost of shipping in your food because you're not growing on that land, from elsewhere, could be much higher. One has to be very careful about these sorts of carbon equations.
Gernot - Absolutely. In some sense that's the bread and butter of what economists do. Figuring out those trade-offs, you can go back to philosopher Mick Jagger on this one as you can't always get what you want. Yes, there are trade-offs and don't get me started on subsidies for biofuels in the US. Why do we do that? Because the presidential primary starts in Iowa and there's a lot of corn in Iowa, that's why we will never get rid of those misguided subsidies. There's lots of politics here. No, we should not now stop producing food and only have solar panels everywhere, but that said, there's plenty of marginal lands where we are not growing food where very little else happens, where a solar farm would make sense.

51:34 - Herbarium: scrap books of potential medicines
Herbarium: scrap books of potential medicines
Emory University ethnobotanist, herbarium curator and intrepid explorer, Cassandra Quave tells describes her precious records...
Chris - I'm intrigued by your role as someone who runs a herbarium because there was a great story a few years back where scientists worked out from stored specimens, what caused the Irish potato famine. That was done in the Southeast of the UK. They happened to have a specimen and they could work out what caused something that happened historically over 150 years ago. Are you able to delve into what you've got in herbaria to find treatments, or are you having to actually get out there in the wild and look at the real deal?
Cassandra - That's a great question. First let me just explain what a herbarium is because I think sometimes people envision this lush tropical greenhouse, and in fact, a herbarium is a museum of dead plants that are pressed to paper. I always tell my friends, I have a brown thumb. I'm very good at finding and killing plants and drawing them and gluing them to paper, but herbaria are incredibly important as records of life at specific points in time in specific places. Your mention of the Irish potato famine, those specimens are a record of what the crops looked like, what other microbes may have been on the leaves at that time. We now have more advanced chemistry tools, we can look into the chemistry of fragments of those specimens. In reality, when it comes to drug discovery of looking for new molecules we generally need a lot more material than what you would find just on a press specimen. We're talking about 40 grams of dried leaves to really kick start the search process. Those herbarium specimens are incredibly important for authenticating and really having a documented record of which plants we're working on. This is a big issue in the literature because there are lots of publications on plants, including clinical studies on botanical remedies and some of the reasons we get into trouble with seeing different outcomes is often these studies don't really have rigorous authentication of their materials, and the chemistry even of related species can be quite different. The chemistry of the same species can be different also depending on where it's grown because of the influence of environmental factors. Really controlling for records of what species you're working on and also controlling or characterising the chemistry is really important to that process.
Chris - Going back to something we were talking about earlier with climate change causing loss of plant species, we've also got the technological revolution causing loss of some cultural values. People are increasingly moving to cities and they're abandoning their relationship with nature. Is there anyone out there, or are there projects out there to try and capture that vast local knowledge before it is lost to the technological revolution so that we've got those folklore remedies stored somewhere so that people like you can subsequently follow them up and hopefully find the next blockbuster drug in the future?
Cassandra - I mean, absolutely. That's really the bread and butter of what ethnobotanists do in the field, it's recording traditional knowledge, and sometimes we also call it traditional ecological knowledge, to really document the ways that people use plants in different cultures. We've done a lot of work in the Balkans and had a really nice study a number of years ago where we looked at two different cultures that had two different languages spoken, different practices. We really examined how those cultural lenses influenced the way that they interacted and engaged with plants in the same environment. They're in the same mountain range, relatively close to one another, but don't intermarry. There were huge differences in not only how they named plants in these different languages, but also which plants were used for food and medicine, even in places where you have the same species growing, the historical trajectory, that cultural lens is incredibly important. We're in a race to save that knowledge before it's lost forever. I think about this in the Amazon as well. We have around 400 different distinct tribal groups and each tribal group has its own language way of viewing and understanding the world and different systems of medicine. When those knowledge keepers pass on without sharing that information with apprentices, where there's a break in the oral tradition of teaching the next generation how to use nature as medicine, we're really losing the equivalency of libraries of knowledge. There is an urgent need to record this knowledge and also document and authenticate the plants, as we mentioned before in herbaria, before we run out of time.

55:23 - The invisible universe
The invisible universe
Matthew Bothwell, Camrbidge University's public astronomer, brings space science down to Earth...
Matthew - The quick answer is that there is an awful lot more that we can't see. I'll give you an analogy. The difference in wavelength between the reddest and bluest light we can see is roughly a factor of two. So, the bluest light we can see is around 380 nanometers and then the reddest light we can see is about 740 nanometers. We have this factor of two in wavelength, which is our window that we use to see the world. There's a nice coincidence. A factor of two in wavelength also has meaning in terms of sound. A factor of two in wavelength is one octave. If you think of red light as being middle C and then the bluest light is the C one oxidative higher. That octave is the window that we use to see the world. The full spectrum of light that arrives from the universe is about 65 octaves. That's nine grand pianos standing in the line, right? If you imagine all those pianos being played at once and you could only hear one central octave you would miss almost all of the music. That's the problem with astronomy. If you're only looking with our eyes you're missing almost all of the information that's out there
Chris - What's the solution?
Matthew - The solution is to build instruments that can see in different wavelengths. I'll give you an example from my research. There's a species of galaxy in the early universe that is completely invisible if you look using the light we can see with our eyes, what we call optical light. These galaxies are amazing. They're some of the most powerful factories for stars in the whole of the universe, but they're all cocooned away behind shrouds of dust. No light leaks out, but we go to long wavelengths, like infrared and radio waves and we can see through the dust and discover these amazing galaxies. If you were a biologist and you could just strap on a pair of infrared goggles and see brand new invisible species, that would be the discovery of a lifetime. That's kind of what we can do with astronomy. We can go to these other wavelengths and see things in the universe that we just didn't know existed.
Chris - Is this big business now, is this really the next step forward? People are beginning to explore new wavelengths, new regimes, to see through these dusty shrouds that we couldn't penetrate previously.
Matthew - It is absolutely big business. Yes. All of modern astronomy is this multi-wavelength affair. The job of a modern astronomist is to collect all of the data from across the spectrum and combine it all into one big synthesised picture of what the universe is doing. There's even stuff beyond that. Modern cosmology tells us that most of the universe is invisible, full stop, it's made of dark matter dark energy that we can't see in any wavelength. It's big business to understand the things that we can see and then it's even bigger business in the future to figure out the rest of the universe that is, at the moment, completely baffling.
Chris - Is it not also true that a sizable chunk of the universe is off limits to us anyway? It's growing and growing away from us faster than we could ever reach. Even if we could travel at the speed of light we could never get there and enjoy the restaurant at the end of the universe because it would have invented more universe by the time we got there.
Matthew - Yeah. That's exactly true. There's this thing called the cosmic horizon, which is a bit like the horizon on earth. It's the bit that you can't see. It's a very long way away. It's about 46 and a half billion light years to the edge. Beyond that we just don't know, right... The universe might be infinite or it might have an edge. We might never know because we are trapped inside our cosmic horizon.










Comments
Add a comment